Mutant IDH1-driven cellular transformation increases RAD51-mediated homologous recombination and temozolomide resistance
- PMID: 25035396
- PMCID: PMC4154998
- DOI: 10.1158/0008-5472.CAN-14-0924
Mutant IDH1-driven cellular transformation increases RAD51-mediated homologous recombination and temozolomide resistance
Abstract
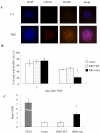
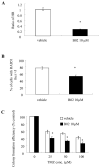
Isocitrate dehydrogenase 1 (IDH1) mutations occur in most lower grade glioma and not only drive gliomagenesis but are also associated with longer patient survival and improved response to temozolomide. To investigate the possible causative relationship between these events, we introduced wild-type (WT) or mutant IDH1 into immortalized, untransformed human astrocytes, then monitored transformation status and temozolomide response. Temozolomide-sensitive parental cells exhibited DNA damage (γ-H2AX foci) and a prolonged G2 cell-cycle arrest beginning three days after temozolomide (100 μmol/L, 3 hours) exposure and persisting for more than four days. The same cells transformed by expression of mutant IDH1 exhibited a comparable degree of DNA damage and cell-cycle arrest, but both events resolved significantly faster in association with increased, rather than decreased, clonogenic survival. The increases in DNA damage processing, cell-cycle progression, and clonogenicity were unique to cells transformed by mutant IDH1, and were not noted in cells transformed by WT IDH1 or an oncogenic form (V12H) of Ras. Similarly, these effects were not noted following introduction of mutant IDH1 into Ras-transformed cells or established glioma cells. They were, however, associated with increased homologous recombination (HR) and could be reversed by the genetic or pharmacologic suppression of the HR DNA repair protein RAD51. These results show that mutant IDH1 drives a unique set of transformative events that indirectly enhance HR and facilitate repair of temozolomide-induced DNA damage and temozolomide resistance. The results also suggest that inhibitors of HR may be a viable means to enhance temozolomide response in IDH1-mutant glioma.
©2014 American Association for Cancer Research.
Figures



References
-
- Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–74. - PubMed
-
- Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–54. - PubMed
-
- Van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1598–1604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

