Requirements for pseudosubstrate arginine residues during autoinhibition and phosphatidylinositol 3,4,5-(PO₄)₃-dependent activation of atypical PKC
- PMID: 25035426
- PMCID: PMC4155669
- DOI: 10.1074/jbc.M114.565671
Requirements for pseudosubstrate arginine residues during autoinhibition and phosphatidylinositol 3,4,5-(PO₄)₃-dependent activation of atypical PKC
Abstract
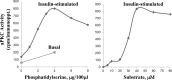
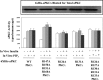
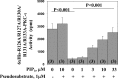
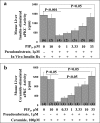
Atypical PKC (aPKC) isoforms are activated by the phosphatidylinositol 3-kinase product phosphatidylinositol 3,4,5-(PO4)3 (PIP3). How PIP3 activates aPKC is unknown. Although Akt activation involves PIP3 binding to basic residues in the Akt pleckstrin homology domain, aPKCs lack this domain. Here we examined the role of basic arginine residues common to aPKC pseudosubstrate sequences. Replacement of all five (or certain) arginine residues in the pseudosubstrate sequence of PKC-ι by site-directed mutagenesis led to constitutive activation and unresponsiveness to PIP3 in vitro or insulin in vivo. However, with the addition of the exogenous arginine-containing pseudosubstrate tridecapeptide to inhibit this constitutively active PKC-ι, PIP3-activating effects were restored. A similar restoration of responsiveness to PIP3 was seen when exogenous pseudosubstrate was used to inhibit mouse liver PKC-λ/ζ maximally activated by insulin or ceramide and a truncated, constitutively active PKC-ζ mutant lacking all regulatory domain elements and containing "activating" glutamate residues at loop and autophosphorylation sites (Δ1-247/T410E/T560E-PKC-ζ). NMR studies suggest that PIP3 binds directly to the pseudosubstrate. The ability of PIP3 to counteract the inhibitory effects of the exogenous pseudosubstrate suggests that basic residues in the pseudosubstrate sequence are required for maintaining aPKCs in an inactive state and are targeted by PIP3 for displacement from the substrate-binding site during kinase activation.
Keywords: Diabetes; Insulin Resistance; Phosphatidylinositol Kinase (PI Kinase); Protein Kinase C (PKC); Signal Transduction.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Fields A. P., Frederick L. A., Regala R. P. (2007) Targeting the oncogenic protein kinase C ι for the treatment of cancer. Biochem. Soc. Trans. 23, 1996–2000 - PubMed
-
- Beeson M., Sajan M. P., Dizon M., Grebenev D., Gomez-Daspet J., Miura A., Kanoh Y., Powe J., Bandyopadhyay G., Standaert M. L., Farese R. V. (2003) Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes 52, 1926–1934 - PubMed
-
- Kim Y.-B., Kotani K., Ciaraldi T. P., Henry R. R., Kahn B. B. (2003) Insulin-stimulated protein kinase C-λ/ζ activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes 52, 1935–1942 - PubMed
-
- Sajan M. P., Standaert M. L., Miura A., Bandyopadhyay G., Vollenweider P., Franklin D. M, Lea-Currie R., Farese R. V. (2004) Impaired activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 in cultured pre-adipocyte-derived adipocytes and myotubes of obese subjects. J. Clin. Endocrinol. Metab. 89, 3994–3998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

