Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes
- PMID: 25035494
- PMCID: PMC4224444
- DOI: 10.1126/science.1254198
Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes
Abstract
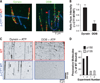
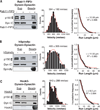
Cytoplasmic dynein is a molecular motor that transports a large variety of cargoes (e.g., organelles, messenger RNAs, and viruses) along microtubules over long intracellular distances. The dynactin protein complex is important for dynein activity in vivo, but its precise role has been unclear. Here, we found that purified mammalian dynein did not move processively on microtubules in vitro. However, when dynein formed a complex with dynactin and one of four different cargo-specific adapter proteins, the motor became ultraprocessive, moving for distances similar to those of native cargoes in living cells. Thus, we propose that dynein is largely inactive in the cytoplasm and that a variety of adapter proteins activate processive motility by linking dynactin to dynein only when the motor is bound to its proper cargo.
Copyright © 2014, American Association for the Advancement of Science.
Figures


Comment in
-
Cell biology. One, two, three, cytoplasmic dynein is go!Science. 2014 Jul 18;345(6194):271-2. doi: 10.1126/science.1257245. Epub 2014 Jul 17. Science. 2014. PMID: 25035476 No abstract available.
-
Traffic control: adaptor proteins guide dynein-cargo takeoff.EMBO J. 2014 Sep 1;33(17):1845-6. doi: 10.15252/embj.201489450. Epub 2014 Jul 24. EMBO J. 2014. PMID: 25061224 Free PMC article.
-
Backseat drivers: Regulation of dynein motility.Cell Res. 2014 Dec;24(12):1385-6. doi: 10.1038/cr.2014.115. Epub 2014 Aug 22. Cell Res. 2014. PMID: 25145357 Free PMC article.
-
Sorting out microtubule-based transport.Nat Rev Mol Cell Biol. 2021 Feb;22(2):73. doi: 10.1038/s41580-020-00320-y. Nat Rev Mol Cell Biol. 2021. PMID: 33288890 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

