Cardiac applications of optogenetics
- PMID: 25035999
- PMCID: PMC4253675
- DOI: 10.1016/j.pbiomolbio.2014.07.001
Cardiac applications of optogenetics
Abstract
In complex multicellular systems, such as the brain or the heart, the ability to selectively perturb and observe the response of individual components at the cellular level and with millisecond resolution in time, is essential for mechanistic understanding of function. Optogenetics uses genetic encoding of light sensitivity (by the expression of microbial opsins) to provide such capabilities for manipulation, recording, and control by light with cell specificity and high spatiotemporal resolution. As an optical approach, it is inherently scalable for remote and parallel interrogation of biological function at the tissue level; with implantable miniaturized devices, the technique is uniquely suitable for in vivo tracking of function, as illustrated by numerous applications in the brain. Its expansion into the cardiac area has been slow. Here, using examples from published research and original data, we focus on optogenetics applications to cardiac electrophysiology, specifically dealing with the ability to manipulate membrane voltage by light with implications for cardiac pacing, cardioversion, cell communication, and arrhythmia research, in general. We discuss gene and cell delivery methods of inscribing light sensitivity in cardiac tissue, functionality of the light-sensitive ion channels within different types of cardiac cells, utility in probing electrical coupling between different cell types, approaches and design solutions to all-optical electrophysiology by the combination of optogenetic sensors and actuators, and specific challenges in moving towards in vivo cardiac optogenetics.
Keywords: Cardiomyocytes; Cell delivery; Channelrhodopsin; Fibroblasts; Gene delivery; Optogenetics.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


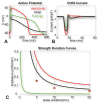

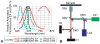


References
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–9. - PubMed
-
- Ambrosi CM, Chen K, Kalra J, Chen X, Zhu W, Yu J, Jiang YP, Lin RZ, Cohen IS, Entcheva E. Book Distribution of donor cells alters energy requirements for optogenetic control of the heart. City: 2013. Distribution of donor cells alters energy requirements for optogenetic control of the heart.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

