Tubular overexpression of gremlin induces renal damage susceptibility in mice
- PMID: 25036148
- PMCID: PMC4103765
- DOI: 10.1371/journal.pone.0101879
Tubular overexpression of gremlin induces renal damage susceptibility in mice
Erratum in
- PLoS One. 2014;9(10):e112003
Abstract
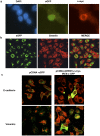
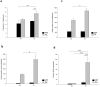
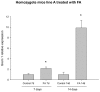
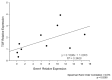
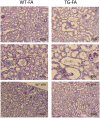
A growing number of patients are recognized worldwide to have chronic kidney disease. Glomerular and interstitial fibrosis are hallmarks of renal progression. However, fibrosis of the kidney remains an unresolved challenge, and its molecular mechanisms are still not fully understood. Gremlin is an embryogenic gene that has been shown to play a key role in nephrogenesis, and its expression is generally low in the normal adult kidney. However, gremlin expression is elevated in many human renal diseases, including diabetic nephropathy, pauci-immune glomerulonephritis and chronic allograft nephropathy. Several studies have proposed that gremlin may be involved in renal damage by acting as a downstream mediator of TGF-β. To examine the in vivo role of gremlin in kidney pathophysiology, we generated seven viable transgenic mouse lines expressing human gremlin (GREM1) specifically in renal proximal tubular epithelial cells under the control of an androgen-regulated promoter. These lines demonstrated 1.2- to 200-fold increased GREM1 expression. GREM1 transgenic mice presented a normal phenotype and were without proteinuria and renal function involvement. In response to the acute renal damage cause by folic acid nephrotoxicity, tubule-specific GREM1 transgenic mice developed increased proteinuria after 7 and 14 days compared with wild-type treated mice. At 14 days tubular lesions, such as dilatation, epithelium flattening and hyaline casts, with interstitial cell infiltration and mild fibrosis were significantly more prominent in transgenic mice than wild-type mice. Tubular GREM1 overexpression was correlated with the renal upregulation of profibrotic factors, such as TGF-β and αSMA, and with increased numbers of monocytes/macrophages and lymphocytes compared to wild-type mice. Taken together, our results suggest that GREM1-overexpressing mice have an increased susceptibility to renal damage, supporting the involvement of gremlin in renal damage progression. This transgenic mouse model could be used as a new tool for enhancing the knowledge of renal disease progression.
Conflict of interest statement
Figures

References
-
- Topol LZ, Bardot B, Zhang Q, Resau J, Huillard E, et al. (2000) Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. J Biol Chem 275: 8785–8793. - PubMed
-
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM (1998) The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1: 673–683. - PubMed
-
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, et al. (2004) Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131: 3401–3410. - PubMed
-
- Roxburgh SA, Murphy M, Pollock CA, Brazil D (2006) Recapitulation of embryological programmes in renal fibrosis–the importance of epithelial cell plasticity and developmental genes. Nephron Physiol 103: 139–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

