Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat
- PMID: 25036185
- PMCID: PMC4103831
- DOI: 10.1371/journal.pone.0102342
Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat
Abstract
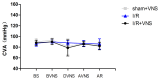
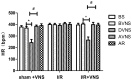
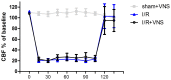
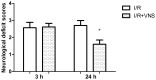
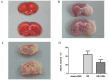
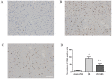
Inflammation and apoptosis play critical roles in the acute progression of ischemic injury pathology. Emerging evidence indicates that vagus nerve stimulation (VNS) following focal cerebral ischemia and reperfusion (I/R) may be neuroprotective by limiting infarct size. However, the underlying molecular mechanisms remain unclear. In this study, we investigated whether the protective effects of VNS in acute cerebral I/R injury were associated with anti-inflammatory and anti-apoptotic processes. Male Sprague-Dawley (SD) rats underwent VNS at 30 min after focal cerebral I/R surgery. Twenty-four h after reperfusion, neurological deficit scores, infarct volume, and neuronal apoptosis were evaluated. In addition, the levels of pro-inflammatory cytokines were detected using enzyme-linked immune sorbent assay (ELISA), and immunofluorescence staining for the endogenous "cholinergic anti-inflammatory pathway" was also performed. The protein expression of a7 nicotinic acetylcholine receptor (a7nAchR), phosphorylated Akt (p-Akt), and cleaved caspase 3 in ischemic penumbra were determined with Western blot analysis. I/R rats treated with VNS (I/R+VNS) had significantly better neurological deficit scores, reduced cerebral infarct volume, and decreased number of TdT mediated dUTP nick end labeling (TUNEL) positive cells. Furthermore, in the ischemic penumbra of the I/R+VNS group, the levels of pro-inflammatory cytokines and cleaved caspase 3 protein were significantly decreased, and the levels of a7nAchR and phosphorylated Akt were significantly increased relative to the I/R alone group. These results indicate that VNS is neuroprotective in acute cerebral I/R injury by suppressing inflammation and apoptosis via activation of cholinergic and a7nAchR/Akt pathways.
Conflict of interest statement
Figures
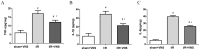

References
-
- Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111: 483–495. - PubMed
-
- Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, et al. (2008) Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke 39: 983–990. - PubMed
-
- Fu SH, Zhang HF, Yang ZB, Li TB, Liu B, et al. (2014) Alda-1 reduces cerebral ischemia/reperfusion injury in rat through clearance of reactive aldehydes. Naunyn Schmiedebergs Arch Pharmacol 387: 87–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

