A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways
- PMID: 25036637
- PMCID: PMC4104544
- DOI: 10.1016/j.cell.2014.05.039
A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways
Abstract
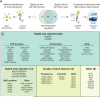
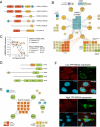
Chaperones are abundant cellular proteins that promote the folding and function of their substrate proteins (clients). In vivo, chaperones also associate with a large and diverse set of cofactors (cochaperones) that regulate their specificity and function. However, how these cochaperones regulate protein folding and whether they have chaperone-independent biological functions is largely unknown. We combined mass spectrometry and quantitative high-throughput LUMIER assays to systematically characterize the chaperone-cochaperone-client interaction network in human cells. We uncover hundreds of chaperone clients, delineate their participation in specific cochaperone complexes, and establish a surprisingly distinct network of protein-protein interactions for cochaperones. As a salient example of the power of such analysis, we establish that NUDC family cochaperones specifically associate with structurally related but evolutionarily distinct β-propeller folds. We provide a framework for deciphering the proteostasis network and its regulation in development and disease and expand the use of chaperones as sensors for drug-target engagement.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

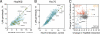



References
-
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. - PubMed
-
- Briknarová K, Takayama S, Homma S, Baker K, Cabezas E, Hoyt DW, Li Z, Satterthwait AC, Ely KR. BAG4/SODD protein contains a short BAG domain. J Biol Chem. 2002;277:31172–31178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

