Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium
- PMID: 25036710
- PMCID: PMC4109553
- DOI: 10.1172/JCI73932
Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium
Abstract
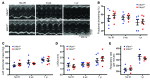
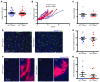
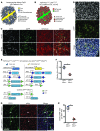
Cells isolated from patients with ataxia telangiectasia are exquisitely sensitive to ionizing radiation. Kinase inhibitors of ATM, the gene mutated in ataxia telangiectasia, can sensitize tumor cells to radiation therapy, but concern that inhibiting ATM in normal tissues will also increase normal tissue toxicity from radiation has limited their clinical application. Endothelial cell damage can contribute to the development of long-term side effects after radiation therapy, but the role of endothelial cell death in tumor response to radiation therapy remains controversial. Here, we developed dual recombinase technology using both FlpO and Cre recombinases to generate primary sarcomas in mice with endothelial cell-specific deletion of Atm to determine whether loss of Atm in endothelial cells sensitizes tumors and normal tissues to radiation. Although deletion of Atm in proliferating tumor endothelial cells enhanced the response of sarcomas to radiation, Atm deletion in quiescent endothelial cells of the heart did not sensitize mice to radiation-induced myocardial necrosis. Blocking cell cycle progression reversed the effect of Atm loss on tumor endothelial cell radiosensitivity. These results indicate that endothelial cells must progress through the cell cycle in order to be radiosensitized by Atm deletion.
Figures



Comment in
-
Radiation and ATM inhibition: the heart of the matter.J Clin Invest. 2014 Aug;124(8):3289-91. doi: 10.1172/JCI77195. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036714 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

