Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs
- PMID: 25036727
- PMCID: PMC4103851
- DOI: 10.1371/journal.pone.0102077
Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs
Abstract
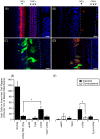
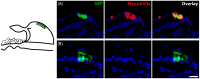
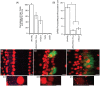
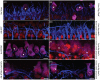
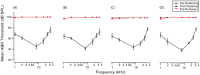
The degeneration of hair cells in the mammalian cochlea results in permanent sensorineural hearing loss. This study aimed to promote the regeneration of sensory hair cells in the mature cochlea and their reconnection with auditory neurons through the introduction of ATOH1, a transcription factor known to be necessary for hair cell development, and the introduction of neurotrophic factors. Adenoviral vectors containing ATOH1 alone, or with neurotrophin-3 and brain derived neurotrophic factor were injected into the lower basal scala media of guinea pig cochleae four days post ototoxic deafening. Guinea pigs treated with ATOH1 gene therapy, alone, had a significantly greater number of cells expressing hair cell markers compared to the contralateral non-treated cochlea when examined 3 weeks post-treatment. This increase, however, did not result in a commensurate improvement in hearing thresholds, nor was there an increase in synaptic ribbons, as measured by CtBP2 puncta after ATOH1 treatment alone, or when combined with neurotrophins. However, hair cell formation and synaptogenesis after co-treatment with ATOH1 and neurotrophic factors remain inconclusive as viral transduction was reduced due to the halving of viral titres when the samples were combined. Collectively, these data suggest that, whilst ATOH1 alone can drive non-sensory cells towards an immature sensory hair cell phenotype in the mature cochlea, this does not result in functional improvements after aminoglycoside-induced deafness.
Conflict of interest statement
Figures


References
-
- Feghali JG, Lefebvre PP, Staecker H, Kopke R, Frenz DA, et al. (1998) Mammalian auditory hair cell regeneration/repair and protection: a review and future directions. Ear Nose Throat J. 77: 276, 280, 282–275. - PubMed
-
- Kong YY, Cruz R, Jones JA, Zeng FG (2004) Music perception with temporal cues in acoustic and electric hearing. Ear Hear 25: 173–185. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, et al. (1999) Math1: an essential gene for the generation of inner ear hair cells. Science 284: 1837–1841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

