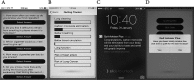
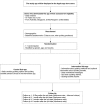
Assessing the effect of an interactive decision-aid smartphone smoking cessation application (app) on quit rates: a double-blind automated randomised control trial protocol
- PMID: 25037644
- PMCID: PMC4120407
- DOI: 10.1136/bmjopen-2014-005371
Assessing the effect of an interactive decision-aid smartphone smoking cessation application (app) on quit rates: a double-blind automated randomised control trial protocol
Abstract
Introduction: In a previous study exploring the feasibility of a smoking cessation application (app), we found that about 77% of the respondents from three countries were ready to quit in the next 30 days without significant differences between countries in terms of age, operating system and number of quitting attempts. However, the efficacy of smartphone apps for smoking cessation has not yet been established. This study tests the efficacy of a smartphone smoking cessation decision-aid app compared with an app that contains only smoking cessation information.
Methods and analysis: This is an automated double-blind, randomised controlled trial of a smoking cessation app that contains the eligibility requirements and baseline questionnaire and will randomise the participants into one of the two subapps (the intervention and the control). Participants will be recruited directly from the Apple app stores in Australia, Singapore, the UK and the USA. Daily smokers aged 18 and above will be randomised into one of the subapps after completing the baseline questionnaire. Abstinence rates will be measured at 10 days, 1 month, 3 months and 6 months, with the 1-month follow-up abstinence rate as the primary outcome. Logistic regression mixed models will be used to analyse the primary outcome.
Ethics and dissemination: This study was approved by the University of Sydney's Human Ethics Committee. The results of the trial will be published in peer-reviewed journals according to the CONSORT statement.
Trial registration number: Australian New Zealand ClinicalTrial RegistryACTRN12613000833763.
Keywords: PREVENTIVE MEDICINE; PUBLIC HEALTH.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- BinDhim NF, Freeman B, Trevena L. Pro-smoking apps for smartphones: the latest vehicle for the tobacco industry? Tob Control 2014;23:e4. - PubMed
-
- Mackay M. Australian mobile phone lifestyle index. 2013. http://www.aimia.com.au/enews/2013/Events/AMPLI%202013/Ampli%202013%20Re... (accessed 19 Aug 2013).
-
- Blackbox Research. Smartphones in Singapore: A Whitepaper Secondary Smartphones in Singapore: A Whitepaper 2013. http://www.blackbox.com.sg/wp/wp-content/uploads/2012/05/Blackbox-YKA-Wh...
-
- Deloitte. Secondary Exponential rise in consumers’ adoption of the smartphone—can retailers keep pace? 2013. http://www.deloitte.com/view/en_GB/uk/61d116116757f310VgnVCM1000003256f7...
-
- Nielsenwire. Consumer Electronics Ownership Blasts Off in 2013. Secondary Consumer Electronics Ownership Blasts Off in 20132013. http://www.nielsen.com/us/en/newswire/2013/consumer-electronics-ownershi...
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical