Tcf3 expression marks both stem and progenitor cells in multiple epithelia
- PMID: 25038042
- PMCID: PMC4197553
- DOI: 10.1242/dev.106989
Tcf3 expression marks both stem and progenitor cells in multiple epithelia
Abstract
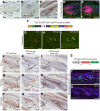
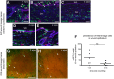
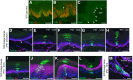
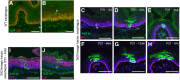
The Lef/Tcf-family transcription factor Tcf3 has important roles in development, stem cell function and malignancy. Previous gain- and loss-of-function studies have suggested that Tcf3 is a mediator of self-renewal and an undifferentiated state in stem and progenitor cells in skin, but little is known of its role in other postnatal tissues. Here, we explore the distribution and behavior of Tcf3-expressing cells in several adult tissues using a novel Tcf3-CreER knock-in mouse model. By lineage tracing in dorsal skin, we verify that Tcf3-expressing cells in the hair follicle bulge are self-renewing stem cells with multilineage potential. We then demonstrate, for the first time, the presence of Tcf3-expressing cells in the basal layer of several other stratified epithelia, including the paw skin, tongue and esophagus. By lineage tracing, we demonstrate that the Tcf3-expressing population in these tissues includes persistent stem cells, transient progenitors and cells undergoing active differentiation. Our observations here suggest that the role of Tcf3 in cell-fate decision is more complex than previously appreciated and is highly dependent on cellular context.
Keywords: Adult stem cells; Lineage tracing; Mouse; Tcf transcription factors; Transcription factor 7-like 1 protein.
© 2014. Published by The Company of Biologists Ltd.
Figures

References
-
- DasGupta R., Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557-4568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

