Using transcriptional control to increase titers of secreted heterologous proteins by the type III secretion system
- PMID: 25038096
- PMCID: PMC4178670
- DOI: 10.1128/AEM.01330-14
Using transcriptional control to increase titers of secreted heterologous proteins by the type III secretion system
Abstract
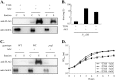
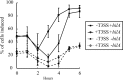
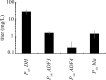
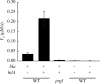
The type III secretion system (T3SS) encoded at the Salmonella pathogenicity island 1 (SPI-1) locus secretes protein directly from the cytosol to the culture media in a concerted, one-step process, bypassing the periplasm. While this approach is attractive for heterologous protein production, product titers are too low for many applications. In addition, the expression of the SPI-1 gene cluster is subject to native regulation, which requires culturing conditions that are not ideal for high-density growth. We used transcriptional control to increase the amount of protein that is secreted into the extracellular space by the T3SS of Salmonella enterica. The controlled expression of the gene encoding SPI-1 transcription factor HilA circumvents the requirement of endogenous induction conditions and allows for synthetic induction of the secretion system. This strategy increases the number of cells that express SPI-1 genes, as measured by promoter activity. In addition, protein secretion titer is sensitive to the time of addition and the concentration of inducer for the protein to be secreted and SPI-1 gene cluster. Overexpression of hilA increases secreted protein titer by >10-fold and enables recovery of up to 28±9 mg/liter of secreted protein from an 8-h culture. We also demonstrate that the protein beta-lactamase is able to adopt an active conformation after secretion, and the increase in secreted titer from hilA overexpression also correlates to increased enzyme activity in the culture supernatant.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

