Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells
- PMID: 25038144
- PMCID: PMC4166745
- DOI: 10.1152/ajpheart.00308.2014
Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells
Abstract
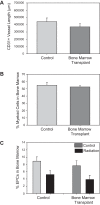
Whole brain radiation therapy (WBRT) induces profound cerebral microvascular rarefaction throughout the hippocampus. Despite the vascular loss and localized cerebral hypoxia, angiogenesis fails to occur, which subsequently induces long-term deficits in learning and memory. The mechanisms underlying the absence of vessel recovery after WBRT are unknown. We tested the hypotheses that vascular recovery fails to occur under control conditions as a result of loss of angiogenic drive in the circulation, chronic tissue inflammation, and/or impaired endothelial cell production/recruitment. We also tested whether systemic hypoxia, which is known to promote vascular recovery, reverses these chronic changes in inflammation and endothelial cell production/recruitment. Ten-week-old C57BL/6 mice were subjected to a clinical series of fractionated WBRT: 4.5-Gy fractions 2 times/wk for 4 wk. Plasma from radiated mice increased in vitro endothelial cell proliferation and adhesion compared with plasma from control mice, indicating that WBRT did not suppress the proangiogenic drive. Analysis of cytokine levels within the hippocampus revealed that IL-10 and IL-12(p40) were significantly increased 1 mo after WBRT; however, systemic hypoxia did not reduce these inflammatory markers. Enumeration of endothelial progenitor cells (EPCs) in the bone marrow and circulation indicated that WBRT reduced EPC production, which was restored with systemic hypoxia. Furthermore, using a bone marrow transplantation model, we determined that bone marrow-derived endothelial-like cells home to the hippocampus after systemic hypoxia. Thus, the loss of production and homing of EPCs have an important role in the prolonged vascular rarefaction after WBRT.
Keywords: angiogenesis; cytokines; endothelial progenitor cells; hypoxia; radiation therapy.
Copyright © 2014 the American Physiological Society.
Figures






References
-
- Akiyama K, Tanaka R, Sato M, Takeda N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir (Tokyo) 41: 590–598, 2001 - PubMed
-
- Angiolillo AL, Sgadari C, Tosato G. A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann NY Acad Sci 795: 158–167, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

