A methodology for a minimum data set for rare diseases to support national centers of excellence for healthcare and research
- PMID: 25038198
- PMCID: PMC4433369
- DOI: 10.1136/amiajnl-2014-002794
A methodology for a minimum data set for rare diseases to support national centers of excellence for healthcare and research
Abstract
Background: Although rare disease patients make up approximately 6-8% of all patients in Europe, it is often difficult to find the necessary expertise for diagnosis and care and the patient numbers needed for rare disease research. The second French National Plan for Rare Diseases highlighted the necessity for better care coordination and epidemiology for rare diseases. A clinical data standard for normalization and exchange of rare disease patient data was proposed. The original methodology used to build the French national minimum data set (F-MDS-RD) common to the 131 expert rare disease centers is presented.
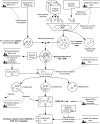
Methods: To encourage consensus at a national level for homogeneous data collection at the point of care for rare disease patients, we first identified four national expert groups. We reviewed the scientific literature for rare disease common data elements (CDEs) in order to build the first version of the F-MDS-RD. The French rare disease expert centers validated the data elements (DEs). The resulting F-MDS-RD was reviewed and approved by the National Plan Strategic Committee. It was then represented in an HL7 electronic format to maximize interoperability with electronic health records.
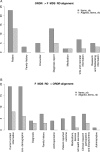
Results: The F-MDS-RD is composed of 58 DEs in six categories: patient, family history, encounter, condition, medication, and questionnaire. It is HL7 compatible and can use various ontologies for diagnosis or sign encoding. The F-MDS-RD was aligned with other CDE initiatives for rare diseases, thus facilitating potential interconnections between rare disease registries.
Conclusions: The French F-MDS-RD was defined through national consensus. It can foster better care coordination and facilitate determining rare disease patients' eligibility for research studies, trials, or cohorts. Since other countries will need to develop their own standards for rare disease data collection, they might benefit from the methods presented here.
Keywords: common data elements; interoperability; metadata; minimum data set; national health program; rare diseases.
© The Author 2014. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For Permissions, please email: journals.permissions@oup.comFor numbered affiliations see end of article.
Figures
References
-
- Orphanet knowledge base for rare diseases. http://www.orpha.net (accessed 1 Jan 2014).
-
- EURORDIS. Eurordis Position Paper on the WHO Report on Priority Medicines for Europe and the World 1. The inconsistency used throughout the Report when addressing the issue of Rare Diseases. 2000. http://www.eurordis.org/IMG/pdf/eurordis_position_WHO__priority_medicine...
-
- van Weely S, Leufkens HGM. Orphan diseases. http://www.pharmaceuticalpolicy.nl/Publications/Reports/7.5Orphandisease....
-
- French health ministry information website on rare diseases. http://www.sante.gouv.fr/les-maladies-rares-qu-est-ce-que-c-est.html (accessed 1 Jan 2014).
-
- EUCERD. Report on the state of the art of rare disease activities in Europe of the European Union committee of experts on rare diseases PART I: overview on rare diseases. 2012. http://www.eucerd.eu/?post_type=document&p=1378
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

