Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection
- PMID: 25038354
- PMCID: PMC4997358
- DOI: 10.1001/jama.2014.7734
Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection
Erratum in
- JAMA. 2014 Nov 12;312(18):1932
Abstract
Importance: Treatment of hepatitis C virus (HCV) infection in patients also infected with human immunodeficiency virus (HIV) has been limited due to drug interactions with antiretroviral therapies (ARTs) and the need to use interferon.
Objective: To determine the rates of HCV eradication (sustained virologic response [SVR]) and adverse events in patients with HCV-HIV coinfection receiving sofosbuvir and ribavirin treatment.
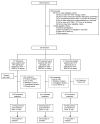
Design, setting, and participants: Open-label, nonrandomized, uncontrolled phase 3 trial conducted at 34 treatment centers in the United States and Puerto Rico (August 2012-November 2013) evaluating treatment with sofosbuvir and ribavirin among patients with HCV genotypes 1, 2, or 3 and concurrent HIV. Patients were required to be receiving ART with HIV RNA values of 50 copies/mL or less and a CD4 T-cell count of more than 200 cells/μL or to have untreated HIV infection with a CD4 T-cell count of more than 500 cells/μL. Of the treatment-naive patients, 114 had HCV genotype 1 and 68 had HCV genotype 2 or 3, and 41 treatment experienced participants who had been treated with peginterferon-ribavirin had HCV genotype 2 or 3, for a total of 223 participants.
Interventions: Treatment-naive patients with HCV genotype 2 or 3 received 400 mg of sofosbuvir and weight-based ribavirin for 12 weeks and treatment-naive patients with HCV genotype 1 and treatment-experienced patients with HCV genotype 2 or 3 received the same treatment for 24 weeks.
Main outcomes and measures: The primary study outcome was the proportion of patients with SVR (serum HCV <25 copies/mL) 12 weeks (SVR12) after cessation of HCV therapy.
Results: Among treatment-naive participants, 87 patients (76%) of 114 (95% CI, 67%-84%) with genotype 1, 23 patients (88%) of 26 with genotype 2 (95% CI, 70%-985), and 28 patients (67%) of 42 with genotype 3 (95% CI, 51%-80%) achieved SVR12. Among treatment-experienced participants, 22 patients (92%) of 24 with genotype 2 (95% CI, 73%-99%) and 16 patients (94%) of 17 (95% CI, 71%-100%) achieved SVR12. The most common adverse events were fatigue, insomnia, headache, and nausea. Seven patients (3%) discontinued HCV treatment due to adverse events. No adverse effect on HIV disease or its treatment was observed.
Conclusions and relevance: In this open-label, nonrandomized, uncontrolled study, patients with HIV who were coinfected with HCV genotype 1, 2, or 3 who received the oral, interferon-free combination of sofosbuvir and ribavirin for 12 or 24 weeks had high rates of SVR12. Further studies of this oral regimen in diverse populations of coinfected patients are warranted.
Trial registration: clinicaltrials.gov Identifier: NCT01667731.
Figures

Comment in
-
Quantum leaps, microeconomics, and the treatment of patients with hepatitis C and HIV coinfection.JAMA. 2014 Jul 23-30;312(4):347-8. doi: 10.1001/jama.2014.7735. JAMA. 2014. PMID: 25038351 No abstract available.
-
HCV/HIV Coinfection: A New Treatment Paradigm.Gastroenterology. 2015 Jun;148(7):1470-1. doi: 10.1053/j.gastro.2015.04.031. Epub 2015 Apr 30. Gastroenterology. 2015. PMID: 25935524 No abstract available.
References
-
- Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85(1):303–315. - PubMed
-
- Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2013;166(15):1632–1641. - PubMed
-
- Sulkowski M, Pol S, Mallolas J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13(7):597–605. - PubMed
-
- Sulkowski MS, Sherman KE, Dieterich DT, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159(2):86–96. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

