Signatures of protective memory immune responses during hepatitis C virus reinfection
- PMID: 25038432
- PMCID: PMC4170061
- DOI: 10.1053/j.gastro.2014.07.005
Signatures of protective memory immune responses during hepatitis C virus reinfection
Abstract
Background & aims: Development of a vaccine against hepatitis C virus (HCV) has been hindered by our limited understanding of immune correlates of protection during real-life exposure to the virus. We studied the immune response during HCV reinfection.
Methods: We analyzed blood samples from participants in the Montreal Acute Hepatitis C Injection Drug User Cohort Study who were reinfected with HCV from 2009 to 2012. Five patients spontaneously resolved their second infection and 4 developed chronic infections. We monitored the phenotypic and functional dynamics of HCV-specific memory T cell responses in all subjects during natural re-exposure and re-infection.
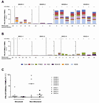
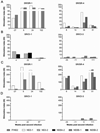
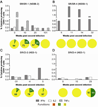
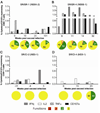
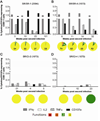
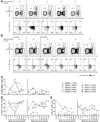
Results: Populations of CD4(+) and CD8(+) T cells with HCV-specific polyfunctional memory were expanded in all 5 individuals who resolved 2 successive HCV infections. We detected CD127(hi) HCV-specific memory CD8(+) T cells before reinfection regardless of a subject's ability to clear subsequent infections. Protection against viral persistence was associated with the expansion of a CD127(neg), PD1(lo) effector memory T cells at the peak of the response. We also observed broadening of T-cell response, indicating generation of de novo T-cell responses. The 4 individuals who failed to clear their subsequent infection had limited expansion of HCV-specific CD4(+) and CD8(+) memory T cells and expressed variable levels of the exhaustion marker PD1 on HCV-specific CD8(+) T cells. Dominant epitope regions of HCV strains isolated from patients with persistent reinfection had sequence variations that were not recognized by the pre-existing memory T cells.
Conclusions: Protection from persistent HCV reinfection depends on the magnitude, breadth, and quality of the HCV-specific memory T-cell response. Sequence homology among viruses and ability of T cells to recognize multiple strains of HCV are critical determinants of protective memory.
Keywords: Cytokines; Immune Regulation; Protective Immunity; Viral Infection.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

