Long-acting beta-agonists plus inhaled corticosteroids safety: a systematic review and meta-analysis of non-randomized studies
- PMID: 25038591
- PMCID: PMC4132190
- DOI: 10.1186/1465-9921-15-83
Long-acting beta-agonists plus inhaled corticosteroids safety: a systematic review and meta-analysis of non-randomized studies
Abstract
Background: Although several systematic reviews investigated the safety of long-acting beta-agonists (LABAs) in asthma, they mainly addressed randomized clinical trials while evidence from non-randomized studies has been mostly neglected. We aim to assess the risk of serious adverse events in adults and children with asthma treated with LABAs and Inhaled Corticosteroids (ICs), compared to patients treated only with ICs, from published non-randomized studies.
Methods: The protocol registration number was CRD42012003387 (http://www.crd.york.ac.uk/Prospero). Literature search for articles published since 1990 was performed in MEDLINE and EMBASE. Two authors selected studies independently for inclusion and extracted the data. A third reviewer resolved discrepancies. To assess the risk of serious adverse events, meta-analyses were performed calculating odds ratio summary estimators using random effect models when heterogeneity was found, and fixed effect models otherwise.
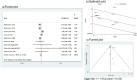
Results: Of 4,415 candidate articles, 1,759 abstracts were reviewed and 220 articles were fully read. Finally, 19 studies met the inclusion criteria. Most of them were retrospective observational cohorts. Sample sizes varied from 50 to 514,216. The meta-analyses performed (69,939-624,303 participants according to the outcome considered) showed that odds ratio of the LABAs and ICs combined treatment when compared with ICs alone was: 0.88 (95% CI 0.69-1.12) for asthma-related hospitalization; 0.75 (95% CI 0.66-0.84) for asthma-related emergency visits; 1.02 (95% CI 0.94-1.10) for systemic corticosteroids; and 0.95 (95% CI 0.9-1.0) for the combined outcome.
Conclusions: Evidence from observational studies shows that the combined treatment of LABAs and ICs is not associated with a higher risk of serious adverse events, compared to ICs alone. Major gaps identified were prospective design, paediatric population and inclusion of mortality as a primary outcome.
Figures




References
-
- Levenson M. Long-acting beta-agonists and adverse asthma events meta-analysis: Briefing package for joint meeting of the Pulmonary-Allergy Drug Advisory Committee and Pediatric Advisory Committee on December 10-11, 2008. 2012. Available from URL: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4398b1-01-FDA.pdf [cited 2012 Oct 4]
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

