Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes
- PMID: 25039804
- PMCID: PMC4291300
- DOI: 10.1111/pedi.12170
Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes
Abstract
Objective: Type 1 diabetes (T1D) results from the inflammatory destruction of pancreatic β-cells. In this study, we investigated the effect of docosahexaenoic acid (DHA) supplementation on stimulated inflammatory cytokine production in white blood cells (WBC) from infants with a high genetic risk for T1D.
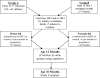
Research design and methods: This was a multicenter, two-arm, randomized, double-blind pilot trial of DHA supplementation, beginning either in the last trimester of pregnancy (41 infants) or in the first 5 months after birth (57 infants). Levels of DHA in infant and maternal red blood cell (RBC) membranes and in breast milk were analyzed by gas chromatography/mass spectrometry. Inflammatory cytokines were assayed from whole blood culture supernatants using the Luminex multiplex assay after stimulation with high dose lipopolysaccharide (LPS), 1 µg/mL.
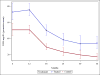
Results: The levels of RBC DHA were increased by 61-100% in treated compared to control infants at ages 6-36 months. There were no statistically significant reductions in production of the inflammatory cytokines, IL-1β, TNFα, or IL-12p40 at any of the six timepoints measured. The inflammatory marker, high-sensitivity C-reactive protein (hsCRP), was significantly lower in breast-fed DHA-treated infants compared to all formula-fed infants at the age of 12 months. Three infants (two received DHA) were removed from the study as a result of developing ≥two persistently positive biochemical islet autoantibodies.
Conclusions: This pilot trial showed that supplementation of infant diets with DHA is safe and fulfilled the pre-study goal of increasing infant RBC DHA levels by at least 20%. Inflammatory cytokine production was not consistently reduced.
Keywords: breast milk DHA; cytokines; docosahexaenoic acid (DHA); red blood cell DHA; type 1 diabetes.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
No conflicts of interest were reported by any co-investigators.
Figures


References
-
- Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001 Oct;44(Suppl 3):B3–B8. - PubMed
-
- Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatr. 2012 Oct;161(4):652–7. - PubMed
-
- Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes–the analysis of the data on published incidence trends. Diabetologia. 1999 Dec;42(12):1395–403. - PubMed
-
- Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996 Jul;45(7):926–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK085476/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK103266/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- UC4 DK117009/DK/NIDDK NIH HHS/United States
- UC4 DK097835/DK/NIDDK NIH HHS/United States
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

