A randomized phase 1 pharmacokinetic trial comparing the potential biosimilar PF-05280014 with trastuzumab in healthy volunteers (REFLECTIONS B327-01)
- PMID: 25041377
- PMCID: PMC4256618
- DOI: 10.1111/bcp.12464
A randomized phase 1 pharmacokinetic trial comparing the potential biosimilar PF-05280014 with trastuzumab in healthy volunteers (REFLECTIONS B327-01)
Abstract
Aims: The pharmacokinetic (PK) similarity between PF-05280014, a proposed trastuzumab biosimilar, trastuzumab sourced from European Union (trastuzumab-EU) or from United States (trastuzumab-US) was evaluated. Safety and immunogenicity were also assessed.
Methods: In this phase 1, double-blind trial (NCT01603264), 105 healthy male volunteers were randomized 1:1:1 to receive a single 6 mg kg(-1) intravenous dose of PF-05280014, trastuzumab-EU, or trastuzumab-US, and evaluated for 70 days. Drug concentration-time data were analyzed by non-compartmental methods. PK similarity for the comparisons of PF-05280014 to each of trastuzumab-EU and trastuzumab-US, and trastuzumab-EU to trastuzumab-US were determined using the standard 80.00% to 125.00% bioequivalence criteria.
Results: Baseline demographics for the 101 subjects evaluable for PK were similar across all arms. The three products exhibited similar PK profiles with target-mediated disposition. The 90% CIs for the ratios of Cmax , AUC (0 , t last) and AUC(0,∞) were within 80.00% to 125.00% for all three pairwise comparisons. Adverse events (AEs) were similar across all arms with treatment-related AEs reported by 71.4%, 68.6% and 65.7% subjects in the PF-05280014, trastuzumab-EU, and trastuzumab-US arms, respectively. The most common AEs were infusion-related reactions, headache, chills, pyrexia and nausea. The AE term 'pyrexia' was numerically greater in the PF-05280014 arm. All post-dose samples, except 1, tested negative for anti-drug antibodies (ADA).
Conclusions: This study demonstrates PK similarity among PF-05280014, trastuzumab-EU and trastuzumab-US. The safety and immunogenicity profiles observed for the three products in this study are consistent with previous reports for trastuzumab.
Keywords: PF-05280014; biosimilar; immunogenicity; pharmacokinetics; safety; trastuzumab.
© 2014 The British Pharmacological Society.
Figures


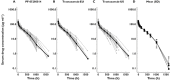
 , median;
, median;  , individual; D: •, PF-02580014; ▪, trastuzumab-EU; ▴, trastuzumab-US
, individual; D: •, PF-02580014; ▪, trastuzumab-EU; ▴, trastuzumab-US
 and (C) AUC(0,∞) of PF-05280014, trastuzumab-EU and trastuzumab-US. AUC(0,∞), area under the concentration–time curve from time 0 extrapolated to infinite time;
and (C) AUC(0,∞) of PF-05280014, trastuzumab-EU and trastuzumab-US. AUC(0,∞), area under the concentration–time curve from time 0 extrapolated to infinite time;  , area under the concentration-time curve from time 0 to last time point of measurable concentration; Cmax, maximum drug concentration; EU, trastuzumab EU; PF, PF-05280014; US, trastuzumab-US
, area under the concentration-time curve from time 0 to last time point of measurable concentration; Cmax, maximum drug concentration; EU, trastuzumab EU; PF, PF-05280014; US, trastuzumab-USReferences
-
- Committee for Medicinal Products for Human Use (CHMP) 2005. Guideline on similar biological medicinal products. European Medicines Agency,. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (last accessed 11 October 2012)
-
- Food and Drug Administration Center for Drug Evaluation and Research. 2012. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. U.S. Department of Health and Human Services,. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... (last accessed 1 March 2012)
-
- Committee for Medicinal Products for Human Use (CHMP) 2012. Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues. European Medicines Agency,. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (last accessed 11 October 2012)
-
- Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. - PubMed
-
- Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28:4–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

