Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance)
- PMID: 25043153
- PMCID: PMC4221473
- DOI: 10.1002/cncr.28892
Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance)
Abstract
Background: Despite targeted antiemetics, data support an unmet need related to the management of delayed nausea and vomiting (NV). Promising pilot data informed this phase III trial evaluating gabapentin for delayed NV from highly emetogenic chemotherapy (HEC).
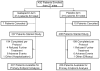
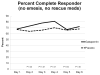
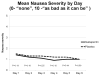
Methods: Participants were randomized to receive prophylactic treatment with 20 mg of dexamethasone and a 5HT3 receptor antagonist (RA) on the day of chemotherapy, followed by gabapentin 300 mg twice a day and dexamethasone (dex) or placebo and dex after HEC. Gabapentin/placebo was started the day of chemotherapy and continued through day 5 for the first chemotherapy cycle, whereas dex was titrated down on days 2-4. The primary end point was complete response (CR), defined as no emesis and no use of rescue medications on days 2-6, using an NV diary. The percentages of those in each group with a CR were compared by Fisher's exact test.
Results: Four hundred thirty patients were enrolled in this study. Forty-seven percent of patients in the gabapentin arm and 41% in the placebo arm had a CR (P = .23). Mean number of emesis episodes was <0.5 daily, and mean nausea severity was < 2 (mild). In both arms, patient satisfaction with NV control was greater than 8 (with 10 being perfectly satisfied). There were no significant differences in unwanted side effects.
Conclusions: In this study, gabapentin did not significantly improve delayed NV. Patients were satisfied with the control of their nausea and vomiting irrespective of arm. The use of a 5HT3 RA and dexamethasone provided good control of nausea and vomiting for most patients.
Keywords: antiemetic therapy; delayed nausea; delayed vomiting; gabapentin; randomized controlled trial.
© 2014 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
Debra L. Barton reports grants from the National Cancer Institute. Jyotsna Fuloria reports grants from the National Cancer Institute, Lisa A. Kottschade reports being on the nurse advisory board of Genentech.
Figures
References
-
- Morrow GR, Hickok JT, Burish TG, et al. Frequency and clinical implications of delayed nausea and delayed emesis. Am J Clin Oncol. 1996;19:199–203. - PubMed
-
- Ihbe-Heffinger A, Ehlken B, Bernard R, et al. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15:526–536. - PubMed
-
- Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. - PubMed
-
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. - PubMed
-
- Tina Shih YC, Xu Y, Elting LS. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer. 2007;110:678–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA035267/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- U10 CA035272/CA/NCI NIH HHS/United States
- CA-35415/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- CA-35269/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA035269/CA/NCI NIH HHS/United States
- CA-37404/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- CA-35195/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- U10 CA035195/CA/NCI NIH HHS/United States
- CA-35103/CA/NCI NIH HHS/United States
- CA-35113/CA/NCI NIH HHS/United States
- U10 CA035101/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- CA-63848/CA/NCI NIH HHS/United States
- U10 CA035415/CA/NCI NIH HHS/United States
- U10 CA063849/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- CA-37417/CA/NCI NIH HHS/United States
- CA-35272/CA/NCI NIH HHS/United States
- CA-35101/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA-35267/CA/NCI NIH HHS/United States
- CA-63849/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

