Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus
- PMID: 25043183
- PMCID: PMC4127309
- DOI: 10.1016/j.celrep.2014.06.024
Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus
Abstract
Although pathogens must infect differentiated host cells that exhibit substantial diversity, documenting the consequences of infection against this heterogeneity is challenging. Single-cell mass cytometry permits deep profiling based on combinatorial expression of surface and intracellular proteins. We used this method to investigate varicella-zoster virus (VZV) infection of tonsil T cells, which mediate viral transport to skin. Our results indicate that VZV induces a continuum of changes regardless of basal phenotypic and functional T cell characteristics. Contrary to the premise that VZV selectively infects T cells with skin trafficking profiles, VZV infection altered T cell surface proteins to enhance or induce these properties. Zap70 and Akt signaling pathways that trigger such surface changes were activated in VZV-infected naive and memory cells by a T cell receptor (TCR)-independent process. Single-cell mass cytometry is likely to be broadly relevant for demonstrating how intracellular pathogens modulate differentiated cells to support pathogenesis in the natural host.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
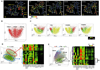

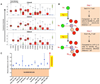

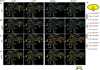
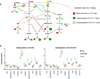

References
-
- Akl H, Badran B, Dobirta G, Manfouo-Foutsop G, Moschitta M, Merimi M, Burny A, Martiat P, Willard-Gallo KE. Progressive loss of CD3 expression after HTLV-I infection results from chromatin remodeling affecting all the CD3 genes and persists despite early viral genes silencing. Virol J. 2007a;4:85. - PMC - PubMed
-
- Akl H, Badran BM, Zein NE, Bex F, Sotiriou C, Willard-Gallo KE, Burny A, Martiat P. HTLV-I infection of WE17/10 CD4+ cell line leads to progressive alteration of Ca2+ influx that eventually results in loss of CD7 expression and activation of an antiapoptotic pathway involving AKT and BAD which paves the way for malignant transformation. Leukemia. 2007b;21:788–796. - PubMed
-
- Alcover A, Alarcon B. Internalization and intracellular fate of TCR-CD3 complexes. Crit Rev Immunol. 2000;20:325–346. - PubMed
-
- Arvin AM, Gilden D. Varicella-zoster Virus. In: Knipe D, Howley P, editors. Fields Virology. Lippincot Williams & Wilkins; 2013.
-
- Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Analytical Chem. 2009;81:6813–6822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA130826/CA/NCI NIH HHS/United States
- PN2 EY018228/EY/NEI NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- 5U54CA143907NIH/CA/NCI NIH HHS/United States
- N01-HV-00242/HV/NHLBI NIH HHS/United States
- P01CA034233-22A1/CA/NCI NIH HHS/United States
- NIH 5-24927/PHS HHS/United States
- U54 CA149145/CA/NCI NIH HHS/United States
- NIH R01 EB 001988/EB/NIBIB NIH HHS/United States
- U54 CA143907/CA/NCI NIH HHS/United States
- P01 CA034233/CA/NCI NIH HHS/United States
- K99GM104148-01/GM/NIGMS NIH HHS/United States
- R01 AI020459/AI/NIAID NIH HHS/United States
- R01 EB001988/EB/NIBIB NIH HHS/United States
- R37 AI020459/AI/NIAID NIH HHS/United States
- U54CA149145/CA/NCI NIH HHS/United States
- 1R01CA130826/CA/NCI NIH HHS/United States
- K99 GM104148/GM/NIGMS NIH HHS/United States
- R01 AI053846/AI/NIAID NIH HHS/United States
- NIH 41000411217/PHS HHS/United States
- AI05346/AI/NIAID NIH HHS/United States
- R00 GM104148/GM/NIGMS NIH HHS/United States
- NIH AI20459/AI/NIAID NIH HHS/United States
- PN2EY018228/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

