Carboxyfullerene neuroprotection postinjury in Parkinsonian nonhuman primates
- PMID: 25043598
- PMCID: PMC4165715
- DOI: 10.1002/ana.24220
Carboxyfullerene neuroprotection postinjury in Parkinsonian nonhuman primates
Abstract
Objective: We evaluated the efficacy of the potent antioxidant C3 to salvage nigrostriatal neuronal function after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) exposure in nonhuman primates. C3 is a first-in-class functionalized water-soluble fullerene that reduces oxygen radical species associated with neurodegeneration in in vitro studies. However, C3 has not been evaluated as a neuroprotective agent in a Parkinson model in vivo.
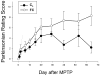
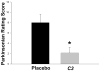
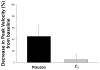
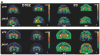
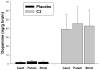
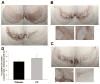
Methods: Macaque fascicularis monkeys were used in a double-blind, placebo-controlled study design. MPTP-lesioned primates were given systemic C3 (n = 8) or placebo (n = 7) for 2 months starting 1 week after MPTP. Outcomes included in vivo behavioral measures of motor parkinsonism using a validated nonhuman primate rating scale, kinematic analyses of peak upper extremity velocity, positron emission tomography imaging of 6-[(18) F]fluorodopa (FD; reflects dopa decarboxylase) and [(11) C]dihydrotetrabenazine (DTBZ; reflects vesicular monoamine transporter type 2), ex vivo quantification of striatal dopamine, and stereologic counts of tyrosine hydroxylase-immunostained neurons in substantia nigra.
Results: After 2 months, C3 -treated monkeys had significantly improved parkinsonian motor ratings, greater striatal FD and DTBZ uptake, and higher striatal dopamine levels. None of the C3 -treated animals developed any toxicity.
Interpretation: Systemic treatment with C3 reduced striatal injury and improved motor function despite administration after the MPTP injury process had begun. These data strongly support further development of C3 as a promising therapeutic agent for Parkinson disease.
© 2014 American Neurological Association.
Figures

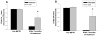
References
-
- Ali SS, Hardt JI, Quick KL, Kim-Han JS, Erlanger BF, Huang TT, Epstein CJ, Dugan LL. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic Biol Med. 2004;37:1191–1202. - PubMed
-
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons Is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. - PubMed
-
- Bezard E, Przedborski S. A tale on animal models of Parkinson’s disease. MovDisord. 2011;26:993–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS039913/NS/NINDS NIH HHS/United States
- R21 AG030320/AG/NIA NIH HHS/United States
- R21AG030320/AG/NIA NIH HHS/United States
- R01NS05425/NS/NINDS NIH HHS/United States
- R01 NS075321/NS/NINDS NIH HHS/United States
- R01 NS050425/NS/NINDS NIH HHS/United States
- R01NS039913/NS/NINDS NIH HHS/United States
- R01 AG033679/AG/NIA NIH HHS/United States
- R01 NS058714/NS/NINDS NIH HHS/United States
- RF1 NS075321/NS/NINDS NIH HHS/United States
- R01NS058714/NS/NINDS NIH HHS/United States
- R01 NS041509/NS/NINDS NIH HHS/United States
- R01NS37688/NS/NINDS NIH HHS/United States
- R01AG033679/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

