Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection
- PMID: 25043633
- PMCID: PMC4311770
- DOI: 10.1002/eji.201344305
Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection
Abstract
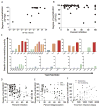
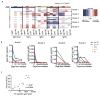
Fc-related antibody activities, such as antibody-dependent cellular cytotoxicity (ADCC), or more broadly, antibody-mediated cellular viral inhibition (ADCVI), play a role in curbing early SIV viral replication, are enriched in human long-term infected nonprogressors, and could potentially contribute to protection from infection. However, little is known about the mechanism by which such humoral immune responses are naturally induced following infection. Here, we focused on the early evolution of the functional antibody response, largely driven by the Fc portion of the antibody, in the context of the evolving binding and neutralizing antibody response, which is driven mainly by the antibody-binding fragment (Fab). We show that ADCVI/ADCC-inducing responses in humans are rapidly generated following acute HIV-1 infection, peak at approximately 6 months postinfection, but decay rapidly in the setting of persistent immune activation, as Fab-related activities persistently increase. Moreover, the loss of Fc activity occurred in synchrony with a loss of HIV-specific IgG3 responses. Our data strongly suggest that Fc- and Fab-related antibody functions are modulated in a distinct manner following acute HIV infection. Vaccination strategies intended to optimally induce both sets of antiviral antibody activities may, therefore, require a fine tuning of the inflammatory response.
Keywords: ADCC; Antibodies; HIV; IgG3.
© 2014 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
All the authors declare no financial or commercial conflict of interest.
Figures



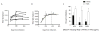
References
-
- Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. - PMC - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

