Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus
- PMID: 25043676
- PMCID: PMC4167938
- DOI: 10.1002/cne.23646
Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus
Abstract
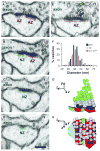
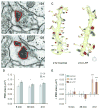
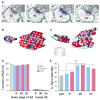
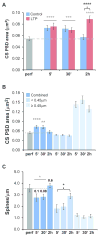
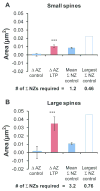
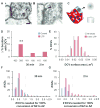
Nascent zones and active zones are adjacent synaptic regions that share a postsynaptic density, but nascent zones lack the presynaptic vesicles found at active zones. Here dendritic spine synapses were reconstructed through serial section electron microscopy (3DEM) and EM tomography to investigate nascent zone dynamics during long-term potentiation (LTP) in mature rat hippocampus. LTP was induced with theta-burst stimulation, and comparisons were made with control stimulation in the same hippocampal slices at 5 minutes, 30 minutes, and 2 hours post-induction and to perfusion-fixed hippocampus in vivo. Nascent zones were present at the edges of ∼35% of synapses in perfusion-fixed hippocampus and as many as ∼50% of synapses in some hippocampal slice conditions. By 5 minutes, small dense-core vesicles known to transport active zone proteins moved into more presynaptic boutons. By 30 minutes, nascent zone area decreased, without significant change in synapse area, suggesting that presynaptic vesicles were recruited to preexisting nascent zones. By 2 hours, both nascent and active zones were enlarged. Immunogold labeling revealed glutamate receptors in nascent zones; however, average distances from nascent zones to docked presynaptic vesicles ranged from 170 ± 5 nm in perfusion-fixed hippocampus to 251 ± 4 nm at enlarged synapses by 2 hours during LTP. Prior stochastic modeling suggests that decrease in glutamate concentration reduces the probability of glutamate receptor activation from 0.4 at the center of release to 0.1 just 200 nm away. Thus, conversion of nascent zones to functional active zones likely requires the recruitment of presynaptic vesicles during LTP.
Keywords: RRID:AB_1769134; RRID:AB_2113447; RRID:nif-0000-23420; RRID:nif-0000-31686; long-term potentiation; postsynaptic density; reconstruction; serial section electron microscopy; synaptic plasticity; synaptic vesicles.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
The authors report no conflicts of interest
The authors declare no competing financial interests.
Figures







References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3(5):445–51. - PubMed
-
- Ahmari SE, Smith SJ. Knowing a nascent synapse when you see it. Neuron. 2002;34(3):333–6. - PubMed
-
- Akins MR, Biederer T. Cell-cell interactions in synaptogenesis. Curr Opin Neurobiol. 2006;16(1):83–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS074644/NS/NINDS NIH HHS/United States
- R01 NS074644/NS/NINDS NIH HHS/United States
- R56 MH095980/MH/NIMH NIH HHS/United States
- NS21184/NS/NINDS NIH HHS/United States
- R01 EB002170/EB/NIBIB NIH HHS/United States
- R01 MH104319/MH/NIMH NIH HHS/United States
- MH095980/MH/NIMH NIH HHS/United States
- R01 NS021184/NS/NINDS NIH HHS/United States
- R37 NS021184/NS/NINDS NIH HHS/United States
- R03 NS071442/NS/NINDS NIH HHS/United States
- NS71442/NS/NINDS NIH HHS/United States
- R01 MH095980/MH/NIMH NIH HHS/United States
- R01 NS033574/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

