Hereditary xerocytosis revisited
- PMID: 25044010
- PMCID: PMC4237618
- DOI: 10.1002/ajh.23799
Hereditary xerocytosis revisited
Abstract
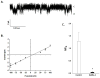
A 21 year old male student presented in 1980 as an Olympic athlete with a 12 year history of jaundice, pallor, and darkened urine induced by the atraumatic exercise of swimming (1). Physical examination at that time was remarkable only for moderate scleral icterus without hepatosplenomegaly. Hematological examination revealed moderate macrocytosis (MCV 102 fL) without anemia (Hct 50%, Hb 17 g/dL, 9% reticulocytes). The peripheral blood smear showed occasional target cells. Red cell osmotic fragility was decreased. Red cell Na content was increased and K content was decreased, with reduced total monovalent ion content. Passive red cell permeability of both Na and K were increased. A supervised 2.5 hr swimming workout increased free plasma Hb from <5 to 45 mg/dL and decreased serum haptoglobin from 25 to 6 mg/dL. The post-exercise urine sediment was remarkable for hemosiderin-laden tubular epithelial cells, without frank hemoglobinuria. The circulating 15 day erythrocyte half-life measured after 6 days without exercise was further shortened to 12 days after resumption of twice-per-day swimming workouts for 1 week. The patient’s red cells were hypersensitive to in vitro shear stress applied by cone-plate viscometer.
Conflict of interest statement
The remaining authors declare no conflicts of interest.
Figures

References
-
- Miller DR, Rickles FR, Lichtman MA, La Celle PL, Bates J, Weed RI. A new variant of hereditary hemolytic anemia with stomatocytosis and erythrocyte cation abnormality. Blood. 1971;38:184–204. - PubMed
-
- Glader BE, Fortier N, Albala MM, Nathan DG. Congenital hemolytic anemia associated with dehydrated erythrocytes and increased potassium loss. N Engl J Med. 1974;291:491–496. - PubMed
-
- Andolfo I, Alper SL, Delaunay J, Auriemma C, Russo R, Asci R, Esposito MR, Sharma AK, Shmukler BE, Brugnara C, et al. Missense mutations in the ABCB6 transporter cause dominant familial pseudohyperkalemia. Am J Hematol. 2013;88:66–72. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
