Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging
- PMID: 25044024
- PMCID: PMC4776755
- DOI: 10.1002/hbm.22588
Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging
Abstract
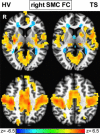
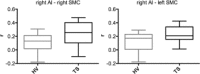
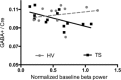
Tourette syndrome (TS) is a neuropsychiatric disorder characterized by motor and vocal tics. Most patients describe uncomfortable premonitory sensations preceding the tics and a subjective experience of increased sensitivity to tactile stimuli. These reports indicate that a sensory processing disturbance is an important component of TS together with motor phenomena. Thus, we focused our investigation on the role of the sensorimotor cortex (SMC) in TS using multimodal neuroimaging techniques. We measured the gamma-aminobutyric acid (GABA)+/Creatine (Cre) ratio in the SMC using GABA (1) H magnetic resonance spectroscopy. We recorded the baseline beta activity in the SMC using magnetoencephalography and correlated GABA+/Cre ratio with baseline beta band power. Finally, we examined the resting state functional connectivity (FC) pattern of the SMC using functional magnetic resonance imaging (fMRI). GABA+/Cre ratio in the SMC did not differ between patients and controls. Correlation between the baseline beta band power and GABA+/Cre ratio was abnormal in patients. The anterior insula showed increased FC with the SMC in patients. These findings suggest that altered limbic input to the SMC and abnormal GABA-mediated beta oscillations in the SMC may underpin some of the sensorimotor processing disturbances in TS and contribute to tic generation.
Keywords: beta oscillations; functional connectivity; functional magnetic resonance imaging; gamma-aminobutyric acid; magnetic resonance spectroscopy; magnetoencephalography; resting state.
Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Figures


References
-
- Barkley RA (2011): Barkley Adult ADHD Rating Scale‐IV (BAARS‐IV). New York: Guilford Press.
-
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M (2006): Neural correlates of tic generation in Tourette syndrome: An event‐related functional MRI study. Brain 129 (Pt 8):2029–2037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

