Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker
- PMID: 25044035
- PMCID: PMC4297605
- DOI: 10.1002/mrm.25358
Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker
Abstract
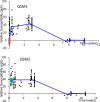
In MRI, the main magnetic field polarizes the electron cloud of a molecule, generating a chemical shift for observer protons within the molecule and a magnetic susceptibility inhomogeneity field for observer protons outside the molecule. The number of water protons surrounding a molecule for detecting its magnetic susceptibility is vastly greater than the number of protons within the molecule for detecting its chemical shift. However, the study of tissue magnetic susceptibility has been hindered by poor molecular specificities of hitherto used methods based on MRI signal phase and T2* contrast, which depend convolutedly on surrounding susceptibility sources. Deconvolution of the MRI signal phase can determine tissue susceptibility but is challenged by the lack of MRI signal in the background and by the zeroes in the dipole kernel. Recently, physically meaningful regularizations, including the Bayesian approach, have been developed to enable accurate quantitative susceptibility mapping (QSM) for studying iron distribution, metabolic oxygen consumption, blood degradation, calcification, demyelination, and other pathophysiological susceptibility changes, as well as contrast agent biodistribution in MRI. This paper attempts to summarize the basic physical concepts and essential algorithmic steps in QSM, to describe clinical and technical issues under active development, and to provide references, codes, and testing data for readers interested in QSM.
Keywords: Bayesian; QSM; calcification; contrast agent; dipole field; dipole kernel; ferritin; gradient echo; hemoglobin; hemorrhage; iron; metabolism; morphology enabled dipole inversion; myelin; oxygen consumption; quantification; quantitative susceptibility mapping.
© 2014 The Authors. Magnetic Resonance in Medicine Published by Wiley Periodicals, Inc. on behalf of International Society of Medicine in Resonance.
Figures
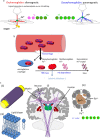

 . At the loop center,
. At the loop center, as
as .(Right) The electron cloud of a molecule polarized by
.(Right) The electron cloud of a molecule polarized by generates magnetic shielding or chemical shift for the observer proton in the molecule and a susceptibility field (in dipole pattern) for the observer proton outside the molecule.
generates magnetic shielding or chemical shift for the observer proton in the molecule and a susceptibility field (in dipole pattern) for the observer proton outside the molecule.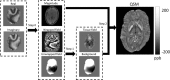

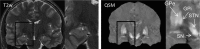
 of the dipole kernel in k-space. (iv) Field map derived at signal-to-noise ratio (SNR) – 20 induced by a point source. (v, vi) Susceptibility in image space obtained by truncated k-space division with the threshold
of the dipole kernel in k-space. (iv) Field map derived at signal-to-noise ratio (SNR) – 20 induced by a point source. (v, vi) Susceptibility in image space obtained by truncated k-space division with the threshold – 0 and 0.1. As a consequence of the dipole kernel zero behavior in the cone surface neighborhood
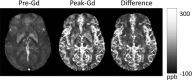
– 0 and 0.1. As a consequence of the dipole kernel zero behavior in the cone surface neighborhood , there is substantial noise propagation from the field measurements into the susceptibility estimate (40), as illustrated in an example of reconstruction by direct division (v and vi). A little noise added in the phase map (peak SNR – 20) leads to a totally corrupted susceptibility image that bears no physical resemblance to the true susceptibility source.
, there is substantial noise propagation from the field measurements into the susceptibility estimate (40), as illustrated in an example of reconstruction by direct division (v and vi). A little noise added in the phase map (peak SNR – 20) leads to a totally corrupted susceptibility image that bears no physical resemblance to the true susceptibility source.

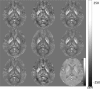
 -space division; TSVD, truncated singular value decomposition; TVSB, total variation using split Bregman.
-space division; TSVD, truncated singular value decomposition; TVSB, total variation using split Bregman.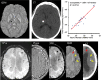



References
-
- Wang Y. 2012. . Principles of Magnetic Resonance Imaging: physics concepts, pulse sequences & biomedical applications. Available at: www.createspace.com/4001776. Accessed December 1, 2013.
-
- de Crespigny AJ, Roberts TP, Kucharcyzk J, Moseley ME. Improved sensitivity to magnetic susceptibility contrast. Magn Reson Med. 1993;30:135–137. - PubMed
-
- Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. - PubMed
-
- Haacke EM, Reichenbach J. Susceptibility weighted imaging in MRI: basic concepts and clinical applications. Hoboken, NJ: Wiley-Blackwell; 2011.
-
- Jackson JD. Classical electrodynamics. 3rd ed. Hoboken, NJ: John Wiley and Son; 1999. pp. 184–188.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

