Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons
- PMID: 25044230
- PMCID: PMC4232589
- DOI: 10.1002/cne.23653
Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons
Abstract
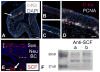
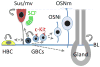
The olfactory epithelium houses chemosensory neurons, which transmit odor information from the nose to the brain. In adult mammals, the olfactory epithelium is a uniquely robust neuroproliferative zone, with the ability to replenish its neuronal and non-neuronal populations due to the presence of germinal basal cells. The stem and progenitor cells of these germinal layers, and their regulatory mechanisms, remain incompletely defined. Here we show that progenitor cells expressing c-Kit, a receptor tyrosine kinase marking stem cells in a variety of embryonic tissues, are required for maintenance of the adult neuroepithelium. Mouse genetic fate-mapping analyses show that embryonically, a c-Kit(+) population contributes to olfactory neurogenesis. In adults under conditions of normal turnover, there is relatively sparse c-Kit(+) progenitor cell (ckPC) activity. However, after experimentally induced neuroepithelial injury, ckPCs are activated such that they reconstitute the neuronal population. There are also occasional non-neuronal cells found to arise from ckPCs. Moreover, the selective depletion of the ckPC population, utilizing temporally controlled targeted diphtheria toxin A expression, results in failure of neurogenesis after experimental injury. Analysis of this model indicates that most ckPCs reside among the globose basal cell populations and act downstream of horizontal basal cells, which can serve as stem cells. Identification of the requirement for olfactory c-Kit-expressing progenitors in olfactory maintenance provides new insight into the mechanisms involved in adult olfactory neurogenesis. Additionally, we define an important and previously unrecognized site of adult c-Kit activity.
Keywords: growth factor; neurogenesis; olfactory epithelium; receptor tyrosine kinase; stem cells.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Figures






References
-
- Bergman U, Ostergren A, Gustafson AL, Brittebo B. Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol. 2002;76(2):104–112. - PubMed
-
- Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122(10):3023–3033. - PubMed
-
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3(1):115–127. - PubMed
-
- Cardiff RD, Hubbard NE, Engelberg JA, Munn RJ, Miller CH, Walls JE, Chen JQ, Velasquez-Garcia HA, Galvez JJ, Bell KJ, Beckett LA, Li YJ, Borowsky AD. Quantitation of fixative-induced morphologic and antigenic variation in mouse and human breast cancers. Lab Invest. 2013;93(4):480–497. - PMC - PubMed
-
- Carr VM, Farbman AI. The dynamics of cell death in the olfactory epithelium. Exp Neurol. 1993;124(2):308–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

