Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies
- PMID: 25045258
- PMCID: PMC4094569
- DOI: 10.2147/COPD.S62502
Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies
Abstract
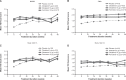
Two replicate, multicenter, randomized, double-blind, placebo-controlled, parallel-group, Phase III studies investigated the long-term efficacy and safety of once-daily olodaterol via Respimat® versus placebo and formoterol over 48 weeks in patients with moderate to very severe chronic obstructive pulmonary disease receiving usual-care background therapy. Patients received once-daily olodaterol 5 or 10 μg, twice-daily formoterol 12 μg, or placebo. Co-primary end points were forced expiratory volume in 1 second (FEV1) area under the curve from 0-3 hours response, FEV1 trough response, and Mahler transition dyspnea index total score after 24 weeks; secondary end points included St George's Respiratory Questionnaire. Overall, 904 (Study 1222.13) and 934 (Study 1222.14) patients received treatment. Olodaterol significantly improved FEV1 area under the curve from 0-3 hours versus placebo in both studies (with olodaterol 5 μg, 0.151 L and 0.129 L; with olodaterol 10 μg, 0.165 L and 0.154 L; for all comparisons P<0.0001) and FEV1 trough responses versus placebo (0.053-0.085 L; P<0.01), as did formoterol. Primary analysis revealed no significant difference in transition dyspnea index focal score for any active treatment versus placebo. Post hoc analysis using pattern mixture modeling (accounting for discontinuations) demonstrated statistical significance for olodaterol versus placebo. St George's Respiratory Questionnaire total score was significantly improved with olodaterol, but not formoterol, versus placebo. No safety signals were identified from adverse-event or other safety data. Once-daily olodaterol 5 μg and 10 μg is efficacious in patients with moderate to very severe chronic obstructive pulmonary disease on usual-care maintenance therapy, with a satisfactory safety profile.
Trial registration: ClinicalTrials.gov NCT00793624 NCT00796653.
Keywords: bronchodilator; chronic obstructive pulmonary disease; dyspnea; long-acting beta2-agonist.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: Updated 2013. [Accessed July 15, 2013]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. - PubMed
-
- Hanania NA, Donohue JF. Pharmacologic interventions in chronic obstructive pulmonary disease. Bronchodilators. Proc Am Thorac Soc. 2007;4(7):526–534. - PubMed
-
- Anderson GP. Formoterol: pharmacology, molecular basis of agonism, and mechanism of long duration of a highly potent and selective beta 2- adrenoceptor agonist bronchodilator. Life Sci. 1993;52(26):2145–2160. - PubMed
-
- Maesen FPV, Smeets JJ, Sledsens TJH, Wald FDM, Cornelissen PJG, Dutch study group Tiotropium bromide, a new long-acting antimuscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD) Eur Respir J. 1995;8(9):1506–1513. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

