Theranostic nanoparticles based on bioreducible polyethylenimine-coated iron oxide for reduction-responsive gene delivery and magnetic resonance imaging
- PMID: 25045265
- PMCID: PMC4099417
- DOI: 10.2147/IJN.S61463
Theranostic nanoparticles based on bioreducible polyethylenimine-coated iron oxide for reduction-responsive gene delivery and magnetic resonance imaging
Abstract
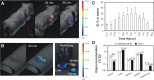
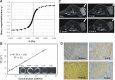
Theranostic nanoparticles based on superparamagnetic iron oxide (SPIO) have a great promise for tumor diagnosis and gene therapy. However, the availability of theranostic nanoparticles with efficient gene transfection and minimal toxicity remains a big challenge. In this study, we construct an intelligent SPIO-based nanoparticle comprising a SPIO inner core and a disulfide-containing polyethylenimine (SSPEI) outer layer, which is referred to as a SSPEI-SPIO nanoparticle, for redox-triggered gene release in response to an intracellular reducing environment. We reveal that SSPEI-SPIO nanoparticles are capable of binding genes to form nano-complexes and mediating a facilitated gene release in the presence of dithiothreitol (5-20 mM), thereby leading to high transfection efficiency against different cancer cells. The SSPEI-SPIO nanoparticles are also able to deliver small interfering RNA (siRNA) for the silencing of human telomerase reverse transcriptase genes in HepG2 cells, causing their apoptosis and growth inhibition. Further, the nanoparticles are applicable as T2-negative contrast agents for magnetic resonance (MR) imaging of a tumor xenografted in a nude mouse. Importantly, SSPEI-SPIO nanoparticles have relatively low cytotoxicity in vitro at a high concentration of 100 μg/mL. The results of this study demonstrate the utility of a disulfide-containing cationic polymer-decorated SPIO nanoparticle as highly potent and low-toxic theranostic nano-system for specific nucleic acid delivery inside cancer cells.
Keywords: MR imaging; RNA interference; SSPEI; disulfide; hTERT; nanoparticles; tumor.
Figures





Similar articles
-
Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer.Int J Nanomedicine. 2017 Jul 26;12:5331-5343. doi: 10.2147/IJN.S137245. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28794626 Free PMC article.
-
Superparamagnetic iron oxide nanoparticles modified with polyethylenimine and galactose for siRNA targeted delivery in hepatocellular carcinoma therapy.Int J Nanomedicine. 2018 Mar 26;13:1851-1865. doi: 10.2147/IJN.S155537. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29618926 Free PMC article.
-
Formulation of glutathione responsive anti-proliferative nanoparticles from thiolated Akt1 siRNA and disulfide-crosslinked PEI for efficient anti-cancer gene therapy.Colloids Surf B Biointerfaces. 2015 Feb 1;126:322-7. doi: 10.1016/j.colsurfb.2014.12.022. Epub 2014 Dec 31. Colloids Surf B Biointerfaces. 2015. PMID: 25576812
-
Application of magnetic nanoparticles to gene delivery.Int J Mol Sci. 2011;12(6):3705-22. doi: 10.3390/ijms12063705. Epub 2011 Jun 7. Int J Mol Sci. 2011. PMID: 21747701 Free PMC article. Review.
-
Bioreducible polycations as shuttles for therapeutic nucleic acid and protein transfection.Antioxid Redox Signal. 2014 Aug 10;21(5):804-17. doi: 10.1089/ars.2013.5714. Epub 2014 Jan 8. Antioxid Redox Signal. 2014. PMID: 24219092 Free PMC article. Review.
Cited by
-
Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances.Mater Today (Kidlington). 2016 Apr;19(3):157-168. doi: 10.1016/j.mattod.2015.08.022. Mater Today (Kidlington). 2016. PMID: 27524934 Free PMC article.
-
Biocompatible Iron Oxide Nanoparticles for Targeted Cancer Gene Therapy: A Review.Nanomaterials (Basel). 2022 Sep 24;12(19):3323. doi: 10.3390/nano12193323. Nanomaterials (Basel). 2022. PMID: 36234452 Free PMC article. Review.
-
siRNA Delivery by Stimuli-Sensitive Nanocarriers.Curr Pharm Des. 2015;21(31):4566-73. doi: 10.2174/138161282131151013190410. Curr Pharm Des. 2015. PMID: 26486143 Free PMC article. Review.
-
Advances in superparamagnetic iron oxide nanoparticles modified with branched polyethyleneimine for multimodal imaging.Front Bioeng Biotechnol. 2024 Jan 25;11:1323316. doi: 10.3389/fbioe.2023.1323316. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38333548 Free PMC article. Review.
-
GE11 peptide modified and reduction-responsive hyaluronic acid-based nanoparticles induced higher efficacy of doxorubicin for breast carcinoma therapy.Int J Nanomedicine. 2016 Oct 7;11:5125-5147. doi: 10.2147/IJN.S113469. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27785019 Free PMC article.
References
-
- Anderson WF. Human gene therapy. Nature. 1998;392:25–30. - PubMed
-
- Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Controlled Release. 2008;126(2):97–110. - PubMed
-
- Li S, Huang L. Non-viral gene therapy: promises and challenges. Gene Ther. 2000;7:31–34. - PubMed
-
- Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):589–593. - PubMed
-
- Patnaik S, Gupta KC. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert Opin Drug Deliv. 2013;10(2):215–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

