Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia
- PMID: 25045434
- PMCID: PMC4102740
- DOI: 10.3802/jgo.2014.25.3.214
Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia
Abstract
Objective: To compare the efficacy of metformin plus megestrol acetate (MA) with that of MA alone for treating endometrial atypical hyperplasia (EAH).
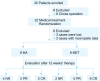
Methods: This pilot study included 16 EAH patients who met at least one metabolic syndrome (MS) criterion and received either adjunctive metformin plus MA (MET group) or MA monotherapy (MA group). Each patient in the MA group received 160 mg of MA daily, whereas patients in the MET group received the same dose of MA plus 0.5 g of metformin thrice daily. Treatment response was assessed by histological examination of dilation and curettage specimens obtained after 12 weeks of therapy.
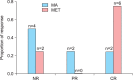
Results: Each group had eight patients, and half of the patients in each group were diagnosed with MS. The complete response (CR) rate was 75% (6/8) in the MET group and 25% (2/8) in the MA group (p=0.105). Complications of MS did not affect the response rates in either group. In the MET group, 75% (3/4) of the patients had CR in the presence or absence of MS. In the MA group, 50% (2/4) of the patients with MS had CR, whereas no patient without MS had CR. No irreversible toxicities were observed.
Conclusion: Metformin plus MA may be a potential alternative therapy for treating EAH, and the MS status of patients may have no effect on the efficacy of metformin plus MA therapy.
Keywords: Endometrial hyperplasia; Megestrol acetate; Metformin.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Conservative treatment for atypical endometrial hyperplasia: what is the most effective therapeutic method?J Gynecol Oncol. 2014 Jul;25(3):164-5. doi: 10.3802/jgo.2014.25.3.164. J Gynecol Oncol. 2014. PMID: 25045426 Free PMC article. No abstract available.
References
-
- Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. - PubMed
-
- Nucera G, Mandato VD, Gelli MC, Palomba S, La Sala GB. Gonadotropin releasing hormone agonist and levonorgestrel-intrauterine device followed by in vitro fertilization program as management strategy for an infertile endometrial cancer patient: a case report. Gynecol Endocrinol. 2013;29:219–221. - PubMed
-
- Jasonni VM, Franceschetti F, Ciotti P, Bulletti C, Vignudelli A, Marabini A, et al. Treatment of endometrial hyperplasia with cyproterone acetate histological and hormonal aspects. Acta Obstet Gynecol Scand. 1986;65:685–687. - PubMed
-
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

