Structure and function of the ependymal barrier and diseases associated with ependyma disruption
- PMID: 25045600
- PMCID: PMC4091052
- DOI: 10.4161/tisb.28426
Structure and function of the ependymal barrier and diseases associated with ependyma disruption
Abstract
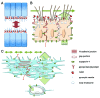
The neuroepithelium is a germinal epithelium containing progenitor cells that produce almost all of the central nervous system cells, including the ependyma. The neuroepithelium and ependyma constitute barriers containing polarized cells covering the embryonic or mature brain ventricles, respectively; therefore, they separate the cerebrospinal fluid that fills cavities from the developing or mature brain parenchyma. As barriers, the neuroepithelium and ependyma play key roles in the central nervous system development processes and physiology. These roles depend on mechanisms related to cell polarity, sensory primary cilia, motile cilia, tight junctions, adherens junctions and gap junctions, machinery for endocytosis and molecule secretion, and water channels. Here, the role of both barriers related to the development of diseases, such as neural tube defects, ciliary dyskinesia, and hydrocephalus, is reviewed.
Keywords: Ependyma; aquaporin 4; astrocyte reaction; cell junctions; cilia; development; hydrocephalus; neural tube defects.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
