Type I and type II Fc receptors regulate innate and adaptive immunity
- PMID: 25045879
- PMCID: PMC7430760
- DOI: 10.1038/ni.2939
Type I and type II Fc receptors regulate innate and adaptive immunity
Abstract
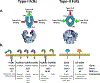
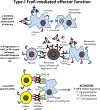
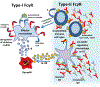
Antibodies produced in response to a foreign antigen are characterized by polyclonality, not only in the diverse epitopes to which their variable domains bind but also in the various effector molecules to which their constant regions (Fc domains) engage. Thus, the antibody's Fc domain mediates diverse effector activities by engaging two distinct classes of Fc receptors (type I and type II) on the basis of the two dominant conformational states that the Fc domain may adopt. These conformational states are regulated by the differences among antibody subclasses in their amino acid sequence and by the complex, biantennary Fc-associated N-linked glycan. Here we discuss the diverse downstream proinflammatory, anti-inflammatory and immunomodulatory consequences of the engagement of type I and type II Fc receptors in the context of infectious, autoimmune, and neoplastic disorders.
Figures


References
-
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews Immunology 2008, 8(1): 34–47. - PubMed
-
- Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev 2010, 236: 265–275. - PubMed
-
- Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. Journal of clinical immunology 2010, 30 Suppl 1: S9–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

