Stable remission and recovery after acute-phase cognitive therapy for recurrent major depressive disorder
- PMID: 25045908
- PMCID: PMC4244279
- DOI: 10.1037/a0037401
Stable remission and recovery after acute-phase cognitive therapy for recurrent major depressive disorder
Abstract
Objective: Continuation-phase cognitive therapy (C-CT) or fluoxetine (FLX) reduces relapse in adults with major depressive disorder (MDD; Jarrett, Minhajuddin, Gershenfeld, Friedman, & Thase, 2013). Among patients at higher risk for relapse, we hypothesized that continuation-phase treatment reduces residual symptoms and facilitates stable remission and recovery.
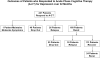
Method: Outpatients (N = 241) with recurrent MDD who responded to acute-phase CT with higher risk for relapse (i.e., had unstable remission defined by any of the last 7 acute-phase scores ≥ 7 using the Hamilton Rating Scale for Depression; Hamilton, 1960) were randomized to 8 months of C-CT, FLX, or pill placebo and followed for 24 additional months. Psychiatric status ratings (Keller et al., 1987) of 1 or 2 (absent or minimal depressive symptoms) for 6 and 35 continuous weeks post-randomization defined stable remission and recovery, respectively.
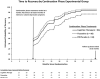
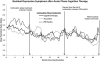
Results: Actuarial estimates of stable remission (97%) and recovery (94%) by the end of follow-up were high and did not differ among groups. Observed (unadjusted) proportions of patients remitting (70%) and recovering (47%) before relapse or attrition were lower. During the continuation phase, C-CT (d = 0.21) and FLX (d = 0.25) patients had significantly lower mean depressive symptoms than did controls, but C-CT and FLX patients did not differ from each other, nor did the 3 experimental groups differ during follow-up.
Conclusion: Many patients who responded to CT with higher relapse risk subsequently remitted and recovered after discontinuation of acute-phase treatment. After discontinuation, C-CT and FLX decreased levels of residual depressive symptoms, but neither significantly increased the likelihood of stable remission or recovery, beyond the moderate to high levels observed among patients who did not relapse.
Figures

Similar articles
-
Predictors of longitudinal outcomes after unstable response to acute-phase cognitive therapy for major depressive disorder.Psychotherapy (Chic). 2015 Jun;52(2):268-77. doi: 10.1037/pst0000021. Psychotherapy (Chic). 2015. PMID: 25985046 Free PMC article. Clinical Trial.
-
Longitudinal social-interpersonal functioning among higher-risk responders to acute-phase cognitive therapy for recurrent major depressive disorder.J Affect Disord. 2016 Jul 15;199:148-56. doi: 10.1016/j.jad.2016.04.017. Epub 2016 Apr 13. J Affect Disord. 2016. PMID: 27104803 Free PMC article. Clinical Trial.
-
Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up.Contemp Clin Trials. 2010 Jul;31(4):355-77. doi: 10.1016/j.cct.2010.04.004. Epub 2010 May 6. Contemp Clin Trials. 2010. PMID: 20451668 Free PMC article. Clinical Trial.
-
Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults.Cochrane Database Syst Rev. 2019 May 20;5(5):CD012855. doi: 10.1002/14651858.CD012855.pub2. Cochrane Database Syst Rev. 2019. PMID: 31106850 Free PMC article.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by
-
Does Symptom Linkage Density Predict Outcomes in Cognitive Therapy for Recurrent Depression?J Psychopathol Behav Assess. 2022 Jun;44(2):469-480. doi: 10.1007/s10862-021-09914-y. Epub 2021 Aug 17. J Psychopathol Behav Assess. 2022. PMID: 35937855 Free PMC article.
-
Could Treatment Matching Patients' Beliefs About Depression Improve Outcomes?Behav Ther. 2019 Jul;50(4):765-777. doi: 10.1016/j.beth.2018.11.007. Epub 2018 Dec 8. Behav Ther. 2019. PMID: 31208686 Free PMC article.
-
Estimating outcome probabilities from early symptom changes in cognitive therapy for recurrent depression.J Consult Clin Psychol. 2019 Jun;87(6):510-520. doi: 10.1037/ccp0000409. Epub 2019 Apr 22. J Consult Clin Psychol. 2019. PMID: 31008632 Free PMC article.
-
Initial Steps to inform selection of continuation cognitive therapy or fluoxetine for higher risk responders to cognitive therapy for recurrent major depressive disorder.Psychiatry Res. 2017 Jul;253:174-181. doi: 10.1016/j.psychres.2017.03.032. Epub 2017 Mar 22. Psychiatry Res. 2017. PMID: 28388454 Free PMC article. Clinical Trial.
-
Cognitive Therapy to Prevent Depressive Relapse in Adults.Curr Opin Psychol. 2015 Aug 1;4:26-31. doi: 10.1016/j.copsyc.2015.01.016. Curr Opin Psychol. 2015. PMID: 25729758 Free PMC article.
References
-
- Allison PD. Survival Analysis Using SAS: A Practical Guide. SAS Institute; Cary, NC: 2010.
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision) Author; Washington, DC: 2000.
-
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. Guilford Press; New York: 1979.
-
- Beevers CG, Rohde P, Stice E, Nolen-Hoeksema S. Recovery from major depressive disorder among female adolescents: A prospective test of the scar hypothesis. Journal of Consulting and Clinical Psychology. 2007;75:888–900. doi:10.1037/0022-006X.75.6.888. - PubMed
-
- Blackburn IM, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. British Journal of Psychiatry. 1997;171:328–334. doi:10.1192/bjp.171.4.328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

