Eliciting neutralizing antibodies with gp120 outer domain constructs based on M-group consensus sequence
- PMID: 25046154
- PMCID: PMC4125429
- DOI: 10.1016/j.virol.2014.06.006
Eliciting neutralizing antibodies with gp120 outer domain constructs based on M-group consensus sequence
Abstract
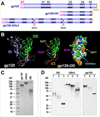
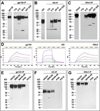
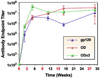
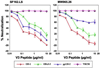
One strategy being evaluated for HIV-1 vaccine development is focusing immune responses towards neutralizing epitopes on the gp120 outer domain (OD) by removing the immunodominant, but non-neutralizing, inner domain. Previous OD constructs have not elicited strong neutralizing antibodies (nAbs). We constructed two immunogens, a monomeric gp120-OD and a trimeric gp120-OD×3, based on an M group consensus sequence (MCON6). Their biochemical and immunological properties were compared with intact gp120. Results indicated better preservation of critical neutralizing epitopes on gp120-OD×3. In contrast to previous studies, our immunogens induced potent, cross-reactive nAbs in rabbits. Although nAbs primarily targeted Tier 1 viruses, they exhibited significant breadth. Epitope mapping analyses indicated that nAbs primarily targeted conserved V3 loop elements. Although the potency and breadth of nAbs were similar for all three immunogens, nAb induction kinetics indicated that gp120-OD×3 was superior to gp120-OD, suggesting that gp120-OD×3 is a promising prototype for further gp120 OD-based immunogen development.
Keywords: HIV-1; Neutralizing antibody; Outer domain; V3; Vaccine; gp120.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, la Peña de AT, Cupo A, Julien J-P, van Gils M, Lee PS, Peng W, Paulson JC, Poignard P, Burton DR, Moore JP, Sanders RW, Wilson IA, Ward AB. Structural Delineation of a Quaternary, Cleavage-Dependent Epitope at the gp41-gp120 Interface on Intact HIV-1 Env Trimers. Immunity. 2014;40:669–680. - PMC - PubMed
-
- Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

