Tiotropium versus placebo for chronic obstructive pulmonary disease
- PMID: 25046211
- PMCID: PMC8934583
- DOI: 10.1002/14651858.CD009285.pub3
Tiotropium versus placebo for chronic obstructive pulmonary disease
Abstract
Background: Tiotropium is an anticholinergic agent which has gained widespread acceptance as a once daily maintenance therapy for symptoms and exacerbations of stable chronic obstructive pulmonary disease (COPD). In the past few years there have been several systematic reviews of the efficacy of tiotropium, however, several new trials have compared tiotropium treatment with placebo, including those of a soft mist inhaler, making an update necessary.
Objectives: To evaluate data from randomised controlled trials (RCTs) comparing the efficacy of tiotropium and placebo in patients with COPD, upon clinically important endpoints.
Search methods: We searched the Cochrane Airways Group's Specialised Register of Trials (CAGR) and ClinicalTrials.gov up to February 2012.
Selection criteria: We included parallel group RCTs of three months or longer comparing treatment with tiotropium against placebo for patients with COPD.
Data collection and analysis: Two review authors independently assessed studies for inclusion and then extracted data on study quality and the outcome results. We contacted study authors and trial sponsors for additional information, and collected information on adverse effects from all trials. We analysed the data using Cochrane Review Manager 5, RevMan 5.2.
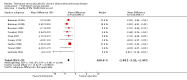
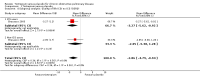
Main results: This review included 22 studies of good methodological quality that had enrolled 23,309 participants with COPD. The studies used similar designs, however, the duration varied from three months to four years. In 19 of the studies, 18 mcg tiotropium once daily via the Handihaler dry powder inhaler was evaluated, and in three studies, 5 or 10 mcg tiotropium once daily via the Respimat soft mist inhaler was evaluated. Compared to placebo, tiotropium treatment significantly improved the mean quality of life (mean difference (MD) -2.89; 95% confidence interval (CI) -3.35 to -2.44), increased the number of participants with a clinically significant improvement (odds ratio (OR) 1.52; 95% CI 1.38 to 1.68), and reduced the number of participants with a clinically significant deterioration (OR 0.65; 95% CI 0.59 to 0.72) in quality of life (measured by the St George's Respiratory Questionnaire (SGRQ)). Tiotropium treatment significantly reduced the number of participants suffering from exacerbations (OR 0.78; 95% CI 0.70 to 0.87). This corresponds to a need to treat 16 patients (95% CI 10 to 36) with tiotropium for a year in order to avoid one additional patient suffering exacerbations, based on the average placebo event rate of 44% from one-year studies. Tiotropium treatment led to fewer hospitalisations due to exacerbations (OR 0.85; 95% CI 0.72 to 1.00), but there was no statistically significant difference in all-cause hospitalisations (OR 1.00; 95% CI 0.88 to 1.13) or non-fatal serious adverse events (OR 1.03; 95% CI 0.97 to 1.10). Additionally, there was no statistically significant difference in all-cause mortality between the tiotropium and placebo groups (Peto OR 0.98; 95% CI 0.86 to 1.11). However, subgroup analysis found a significant difference between the studies using a dry powder inhaler and those with a soft mist inhaler (test for subgroup differences: P = 0.01). With the dry powder inhaler there were fewer deaths in the tiotropium group (Peto OR 0.92; 95% CI 0.80 to 1.05) than in the placebo group (yearly rate 2.8%), but with the soft mist inhaler there were significantly more deaths in the tiotropium group (Peto OR 1.47; 95% CI 1.04 to 2.08) than in the placebo group (yearly rate 1.8%). It is noted that the rates of patients discontinuing study treatment were uneven, with significantly fewer participants withdrawing from tiotropium treatment than from placebo treatment (OR 0.66; 95% CI 0.59 to 0.73). Participants on tiotropium had improved lung function at the end of the study compared with those on placebo (trough forced expiratory volume in one second (FEV1) MD 118.92 mL; 95% CI 113.07 to 124.77).
Authors' conclusions: This review shows that tiotropium treatment was associated with a significant improvement in patients' quality of life and it reduced the risk of exacerbations, with a number needed to treat to benefit (NNTB) of 16 to prevent one exacerbation. Tiotropium also reduced exacerbations leading to hospitalisation but no significant difference was found for hospitalisation of any cause or mortality. Thus, tiotropium appears to be a reasonable choice for the management of patients with stable COPD, as proposed in guidelines. The trials included in this review showed a difference in the risk of mortality when compared with placebo depending on the type of tiotropium delivery device used. However, these results have not been confirmed in a recent trial when 2.5 mcg or 5 mcg of tiotropium via Respimat was used in a direct comparison to the 18 mcg Handihaler.
Conflict of interest statement
None known.
Figures
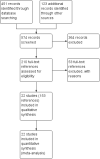
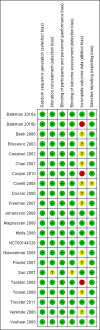































Update of
-
Tiotropium versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD009285. doi: 10.1002/14651858.CD009285.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jul 21;(7):CD009285. doi: 10.1002/14651858.CD009285.pub3. PMID: 22786525 Updated.
References
References to studies included in this review
Bateman 2010a {published and unpublished data}
-
- Bateman ED. A randomised, double‐blind, placebo‐controlled, parallel‐group study to assess long term (one‐year) efficacy and safety of tiotropium inhalation solution 5 mcg (2 puffs of 2.5 mcg) delivered by the Respimat® inhaler in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.372]
-
- Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, et al. A one‐year trial of tiotropium Respimat plus usual therapy in COPD patients. Respiratory Medicine 2010;104(10):1460‐72. [CTG: NCT00387088] - PubMed
Bateman 2010b {published and unpublished data}
-
- Cooper, C. A retrospective collection of vital status and pulmonary medication usage data for patients with chronic obstructive pulmonary disease (COPD) who withdrew prematurely from either of two one‐year trials (205.254, 205.255) of tiotropium inhalation solution delivered by the Respimat® inhaler. www.trials.boehringer‐ingelheim.com (accessed 05 March 2008). [trial number: 205.392]
-
- Singh SD. A randomised, double‐blind, placebo‐controlled, parallel group efficacy and safety comparison of one‐year treatment of two doses (5 μg [2 actuations of 2.5 μg] and 10 μg [2 actuations of 5 μg]) of tiotropium inhalation solution delivered by the Respimat® inhaler in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.255]
-
- Smith D. A Randomised, Double‐Blind Placebo Controlled, Parallel Group Efficacy and Safety Comparison of One‐year Treatment of Two Doses (5 μg [2 actuations of 2.5 μg] and 10 μg [2 actuations of 5 μg]) of Tiotropium Inhalation Solution Delivered by the Respimat® Inhaler in Patients with Chronic Obstructive Pulmonary Disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.254]
Beeh 2006 {published and unpublished data}
-
- Beeh KM, Beier J, Buhl R, Gerken FMN. Efficacy of tiotropium (spriva) in patients with COPD switched from previous treatment with short‐acting anticholinergics [Abstract]. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A113 [Poster J27].
-
- Beeh KM, Beier J, Buhl R, Stark‐Lorenzen P, Gerken F, Metzdorf N, et al. Efficacy of tiotropium bromide (Spiriva) in patients with chronic‐obstructive pulmonary disease (COPD) of different severities [Wirksamkeit von Tiotropiumbromid (Spiriva)bei verschiedenen Schweregraden derchronisch−obstruktiven Lungenerkrankung (COPD)]. Pneumologie 2006;60(6):341‐6. [CTG: NCT00274573] - PubMed
-
- Beeh KM, Beier J, Buhl R, Strak‐Lorenzen P, Gerken F, Metzdorf N. Efficacy of tiotropium in patients with mild to moderate COPD [Abstract]. American Thoracic Society 2004 International Conference; 2004 May 21‐26; Florida, USA. 2004:P513.
-
- Boehringer Ingelheim. Acute and long‐term effects of once daily oral inhalation of tiotropium 18 mcg dry powder inhalation capsules in a placebo controlled parallel group design study in patients with chronic obstructive pulmonary disease (COPD) of different severity. www.trials.boehringer‐ingelheim.com. [trial number: 205.257]
Brusasco 2003 {published and unpublished data}
-
- Bateman ED, Hodder R, Miravitlles M, Lee A, Towse L, Serby C. A comparative trial of tiotropium, salmeterol and placebo: health‐related quality of life. European Respiratory Journal 2001;18(Suppl 33):26.
-
- Bateman ED, Jenkins C, Korducki L, Keston S. Tiotropium (TIO) improves health care resource utilization (HRU) and patient disability in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A111.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders following treatment with tiotropium and salmeterol in patients with COPD [Abstract]. American Thoracic Society 99th International Conference; 2003; May 16 ‐ 21; Seattle. 2003:B024 Poster 420.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. American Thoracic Society Annual Meeting; 2006; May 19‐24; San Diego. 2006:A110 [Poster J10].
Casaburi 2002 {published and unpublished data}
-
- Adams SG, Anzueto A, Briggs DD, Menjoge SS, Kesten S. Tiotropium in COPD patients not previously receiving maintenance respiratory medications. Respiratory Medicine 2006;100(9):1495‐503. - PubMed
-
- Anzueto A, Kesten S. Effects of tiotropium in COPD patients only treated with PRN albuterol [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C47 Poster F81.
-
- Anzueto A, Tashkin D, Menjoge S, Kesten S. One‐year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulmonary Pharmacology and Therapeutics 2005;18(2):75‐81. - PubMed
-
- Casaburi R, Briggs DD Jr, Donohue JF, Serby CW, Menjoge SS, The US Tiotropium Study Group. The spirometric efficacy of once‐daily dosing with tiotropium in stable COPD: a 13‐week multicenter trial. Chest 2000;118(5):1294‐302. - PubMed
-
- Casaburi R, Mahler DA, Jones PW, Wanner A, San Pedro G, Zuwallack RL, et al. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal 2002;19(2):217‐24. - PubMed
Chan 2007 {published and unpublished data}
-
- Chan C. Spiriva assessment of FEV1 (SAFE). The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during long‐term treatment in patients with COPD. A one‐year parallel group, double‐blind, randomised, placebo‐controlled study. Boehringer Ingelheim Clinical Trial Register 2005. [trial number : 205.259]
Cooper 2010 {published and unpublished data}
-
- Cooper C. A randomized, double‐blind, placebo‐controlled two‐year trial to examine the changes in exercise endurance and COPD patients treated with tiotropium (Spiriva® HandiHaler®) 18 μg once daily (EXACTT trial). www.trials.boehringer‐ingelheim.com. [trial number: 205.368]
-
- Cooper CB, Celli B, Wise RA, Jardim JR, Legg D, Guo J, et al. "Treadmill endurance during 2 years" treatment with tiotropium in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2011;183:A4586.
Covelli 2005 {published and unpublished data}
-
- Boehringer Ingelheim. Efficacy and safety (including 24‐hour holter monitoring) of tiotropium inhalation capsules in patients with chronic obstructive pulmonary disease (a 12‐week, parallel group, randomized, placebo‐controlled, double‐blind study). www.trials.boehringer‐ingelheim.com. [trial number: 205.284]
-
- Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S. Absence of electrocardiographic findings and improved function with once‐daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy 2005; Vol. 25, issue 12:1708‐18. [CTG: NCT00239460] - PubMed
-
- Kesten S, Cassino C, Conoscenti C. Absence of electrocardiographic findings with daily tiotropium in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2004;126(4 Suppl):837S. - PubMed
Dusser 2006 {published and unpublished data}
-
- Dusser D. Effect of inhaled tiotropium bromide (18 mcg once daily) on the severity of airflow obstruction during long‐term treatment in patients with moderately severe COPD. Impact on severity and incidence of exacerbations. A one‐year parallel‐group, double‐blind, randomized, placebo‐controlled study. www.trials.boehringer‐ingelheim.com. [trial number: 205.214]
-
- Dusser D, Bravo ML, Lacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. European Respiratory Journal 2006;27(3):547‐55. [CTG: NCT00274014] - PubMed
-
- Dusser D, Bravo ML, Lacono P. Tiotropium reduces health resource utilization associated with COPD exacerbations [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513.
-
- Dusser D, Bravo ML, Lanono P. Tiotropium COPD exacerbations the MISTRAL study [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513.
-
- Dusser D, Viel K, Bravo ML, Lacono P. Tiotropium reduces moderate‐to‐severe exacerbations in COPD patients irrespective of concomitant use of inhaled corticosteroids (ICS) [Abstract]. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A603 [Poster H6].
Freeman 2007 {published and unpublished data}
-
- Freeman D. A randomised, double‐blind, parallel group, 12 week study, comparing the effect of once daily tiotropium lactose capsule with placebo in patients with chronic obstructive pulmonary disease (COPD), naive to anticholinergic agents in addition to receiving their usual COPD care. www.trials.boehringer‐ingelheim.com. [trial number: 205.276]
-
- Freeman D, Sarno M, White L, Lee A, Price D. Spruce: tiotropium in UK primary care [Abstract]. Thorax 2004;59(Suppl II):ii100.
-
- Price D, Sarno M, Lee A, Freeman D. SPiRiva usual carE‐ SPRUCE ‐ tiotropium in a UK primary care COPD population other regular inhaled treatment [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:[A43] [Poster: F26].
Johansson 2008 {published and unpublished data}
-
- Johansson G. A 12‐week, double‐blind, randomised, parallel‐group, multi‐centre study evaluating the efficacy of tiotropium versus placebo in patients with mild COPD according to Swedish guidelines (SPIRIMILD). www.trials.boehringer‐ingelheim.com. [trial number: 205.281]
Magnussen 2008 {published and unpublished data}
-
- Boehringer Ingelheim. A trial evaluating the efficacy and safety of inhaled tiotropium 18 µg qd in patients with COPD and a concomitant diagnosis of asthma. www.clinicaltrials.gov 2005.
-
- Magnussen H. A 12‐week randomised, double blind, placebo‐controlled, parallel group trial evaluating the efficacy and safety of inhaled tiotropium 18 μg q.d. in patients with COPD and a concomitant diagnosis of asthma. www.trials.boehringer‐ingelheim.com. [trial number: 205.301]
-
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respiratory Medicine 2008;102(1):50‐6. [CTG: NCT00152984] - PubMed
-
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S, et al. Spirometric improvements with tiotropium in COPD patients with concomitant asthma [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #A5.
Moita 2008 {published and unpublished data}
-
- Boehringer Ingelheim. Spiriva® assessment of FEV1 ‐ (SAFE‐Portugal). The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during treatment in patients with COPD. A three‐month parallel group, double‐blind, randomised, placebo‐controlled study. www.trials.boehringer‐ingelheim.com. [trial number: 205.282]
-
- Moita J, Barbara C, Cardoso J, Costa R, Sousa M, Ruiz J, et al. Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulmonary Pharmacology and Therapeutics 2008;21(1):146‐51. [CTG: NCT00239408] - PubMed
-
- Moita J, Costa R, Barbara C, Cardosa J, Rulz J, Sousa M. The effect of tiotropium bromide in a stable cohort of COPD patients in Portugal [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1955.
NCT00144326 {published and unpublished data}
-
- García Río, F. A randomised, double‐blind, placebo‐controlled, 12 week trial to evaluate the effect of tiotropium inhalation capsules on the magnitude of exercise, measured using an accelerometer, in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com (accessed 15 Oct 2007). [CTG: NCT00144326; trial number: 205.269]
Niewoehner 2005 {published and unpublished data}
-
- Anonymous. Tiotropium reduces exacerbations in COPD. Pharmaceutical Journal 2004;272(7302):696.
-
- Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE. Premature discontinuation of patients: a potential bias in COPD clinical trials. European Respiratory Journal 2007;30(5):898‐906. - PubMed
-
- Niewoehner D. A randomized, double‐blind, placebo‐controlled, parallel group trial assessing the proportion of patients experiencing an exacerbation and proportion of patients hospitalized for an exacerbation over 6 months during treatment with tiotropium 18 mcg capsule once daily in patients with COPD in a Veterans Affairs setting. www.trials.boehringer‐ingelheim.com. [trial number: 205.266]
-
- Niewoehner D, Gonzalez‐Rothi R, Shigeoka J, Korducki L, Cassino C, Kesten S, et al. Reduced COPD exacerbations in patients without first‐dose peak FEV increases 15% with tiotropium [Abstract] American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego, California. San Diego, 2005:[B93] [Poster: 319].
-
- Niewoehner D, Rice K, Cote C, Korducki L, Cassino C, Kesten S. Characteristics of COPD and response to once‐daily tiotropium in African‐Americans in the VA medical system [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. San Diego, 2005:[A43] [Poster: F41].
Powrie 2007 {published and unpublished data}
-
- Powrie D, Wilkinson T, Donaldson G, Viel K, Jones P, Scrine K, et al. The effect of tiotropium on diary based exacerbation frequency in COPD [Abstract]. American Thoracic Society Annual Meeting; 2006 May 19‐24 2006; San Diego. 2006:A604 [Poster H11].
-
- Powrie DJ, Donaldson GC, Wilkinson TM, Jones P, Viel K, Kesten S, et al. Tiotropium reduces exacerbations and common colds in patients with COPD [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, California, USA. 2007:Poster #A50.
-
- Powrie DJ, Wilkinson TMA, Donaldson GC, Jones P, Scrine K, Viel K, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. European Respiratory Journal 2007;30(3):472‐8. [CTG: NCT00405236] - PubMed
-
- Powrie J, Wilkinson M, Donaldson C, Wedzicha A. Tiotropium reduces subjectively reported sputum production in COPD. European Respiratory Journal 2006;28(Suppl 50):661.
-
- Wedzicha JA. A randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the changes in inflammatory markers and in induced sputum following treatment with tiotropium inhalation capsules 18 μg once daily in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.270]
Sun 2007 {published data only}
-
- Sun LH, Tan Y, Qiao Y, Fang SR, Xie H. Evaluation of clinical effect and safety of tiotropium bromide in treating stable chronic obstructive pulmonary disease. Zhongguo Xinyao yu Linchuang Zazhi 2007;26(5):328‐31.
-
- Yan L, Lu YK. Effect of domestic tiotropium bromide inhalation in patients with COPD at stable stage. Chinese Journal of New Drugs 2010;19(2):127‐129, 138.
Tashkin 2008 {published and unpublished data}
-
- Antoniu SA. UPLIFT Study: The effects of long‐term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2009;10(4):719‐22. - PubMed
-
- Braido F. UPLIFT study: The results and their implications. Multidisciplinary Respiratory Medicine. 2009; Vol. 4, issue Suppl 1:6s‐12s.
-
- Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, et al. Mortality in the 4‐year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009;180(10):948‐55. - PubMed
-
- Celli B, Kesten S, Lystig T, Tashkin DP, Decramer M. Long‐term changes in inspiratory capacity: an analysis from the UPLIFT(R) trial [Abstract]American Thoracic Society (ATS) International Conference; 2010 May 14‐19; New Orleans. 2010, issue Meeting Abstracts:A4482.
-
- Corhay JL, Louis R. The UPLIFT study. Revue Medicale de Liege 2009;64(1):50‐5. - PubMed
Tonnel 2008 {published and unpublished data}
-
- Tonnel AB. Effect of a 9‐month treatment of SPIRIVA® on health related quality of life in patients with chronic obstructive pulmonary disease. Validation of a new HRQoL questionnaire appropriate to common daily practice. (TIPHON Study). www.trials.boehringer‐ingelheim.com. [trial number: 205.256]
-
- Tonnel AB, Bravo ML, Brun M. Clinically significant improvements of health status of COPD patients after 9 months treatment with tiotropium bromide: the TIPHON study [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[B93] [Poster: 302].
-
- Tonnel AB, Perez T, Grosbois JM, Bravo ML, Brun M. Improvement in HRQoL of COPD patients after 9 months treatment with tiotropium bromide: use of a new scale for daily medical practice [Abstract]. European Respiratory Society 15th Annual Congress; 2005 September 17 ‐ 21; Copenhagen. 2005.
Trooster 2011 {published and unpublished data}
-
- Carron K. A 24 week, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy and safety of 18 mcg of tiotropium inhalation capsules administered by handihaler® once‐daily plus prn albuterol (salbutamol) vs. placebo plusprn albuterol (salbutamol) in chronic obstructive pulmonary disease subjects naive to maintenance therapy. www.trials.boehringer‐ingelheim.com. [trial number: 205.365]
-
- Sciurba FC, Siafakas N, Troosters T, Klioze SS, Sutradhar S, Weisman I, et al. The efficacy and safety of tiotropium Handihaler, 18 µg, once daily plus PRN salbutamol versus placebo plus prn salbutamol in COPD subjects naive to maintenance therapy [Abstract]. American Thoracic Society 2011 International Conference; 2011 May 13‐18; Denver. 2011; Vol. 183:A1589.
-
- Troosters T, Weisman I, Dobbels F, Giardino N, Valluri SR. Assessing the impact of tiotropium on lung function and physical activity in GOLD Stage II COPD patients who are naive to maintenance respiratory therapy: a study protocol. The Open Respiratory Medicine Journal 2011;5:1‐9. [CTG: NCT00523991] - PMC - PubMed
Verkinde 2006 {published and unpublished data}
-
- Huchon G. Effect of a 12‐week treatment with inhaled tiotropium (18 mcg once daily) on lung function and static lung volumes in stable, moderate to severe COPD patients. Correlation to dyspnoea scales: a double‐blind, placebo‐controlled, randomized, parallel group study. www.trials.boehringer‐ingelheim.com. [trial number: 205.215]
-
- Huchon G, Verkindre C, Bart F, Aguilaniu B, Guerin JC, Lemerre C, et al. Improvements with tiotropium on endurance measure by the shuttle walking test (SWT) and on health related quality of life (HRQoL) in COPD patients. European Respiratory Society Congress; 2002 September 14‐18; Stockholm. 2002.
-
- Verkindre C, Bart F, Aguilaniu B, Fortin F, Guerin JC, Merre C, et al. The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 2006;73(4):420‐7. - PubMed
Voshaar 2008 {published and unpublished data}
-
- Boehringer Ingelheim. A randomised, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses [5 mcg (2 actuations of 2.5 mcg) and 10 mcg (2 actuations of 5 mcg)] of tiotropium inhalation solution delivered by the Respimat inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.251]
-
- Boehringer Ingelheim. A randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses (5 Mcg and 10 Mcg) of tiotropium inhalation solution delivered by the Respimat inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). www.clinicaltrials.gov 2002; Vol. Sept 2004 End Date. [trial number: 205.252]
-
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomised study of tiotropium Respimat soft mist inhaler versus ipratropium PMDI in chronic obstructive pulmonary disease [Abstract]. Thorax 2006;61(Suppl 2):ii40 [S112]. - PubMed
-
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomized study of tiotropium Respimat Soft Mist inhaler vs. ipratropium pMDI in COPD. Respiratory Medicine 2008;102(1):32‐41. [CTG: NCT00239473; CTG: NCT00240435] - PubMed
References to studies excluded from this review
Ambrosino 2008 {published data only}
Baloira 2005 {published data only}
-
- Baloira A, Vilarino C. Effects of tiotropium bromide in exercise tolerance in patients with chronic obstructive pulmonary disease [Abstract]. European Respiratory Society Congress; 2005 September 17‐21; Copenhagen. 2005; Vol. 26, issue Suppl 49:Abstract No. 1929.
Bedard 2011 {published data only}
-
- Bedard ME, Brouillard C, Pepin V, Milot J, Lacasse Y, Leblanc P, et al. Tiotropium improves walking endurance in chronic obstructive pulmonary disease patients. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A1590.
Calverley 2000 {published data only}
-
- Calverley PMA, Towse LJ, Lee A. The timing of dose and pattern of bronchodilatation of tiotropium (TIO) in stable COPD. European Respiratory Journal 2000;16(Suppl 31):56s.
Casaburi 2005 {published data only}
-
- Casaburi R, Kukafka D, Cooper CB, Kesten S. Improvement in exercise endurance with the combination of tiotropium and rehabilitative exercise training in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:D14.
-
- Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005;127(3):809‐17. - PubMed
-
- Cooper CB, Kukafka D, Casburi R, Kesten S. Tiotropium augments improvements in dyspnea and health‐related quality of life following pulmonary rehabilitation in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C22 Poster 507.
Celli 2002 {published data only}
-
- Celli BR, Zuwallack RL, Wang S, Kesten S. Improvements in inspiratory capacity with tiotropium in patients with COPD [Abstract]. European Respiratory Society Annual Congress 2002. 2002:abstract nr: P3079.
da Fonseca 2010 {published data only}
-
- Fonseca Reis LF, Ventura TG, Jayme S, Bispo P, Cantanhede LA. A role of tiotropium in patients with severe COPD subject to a supervised program of exercises [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P4872].
de Guia 2004 {published data only}
-
- Guia T, Punzal P, Canizares L, Sy‐Naval S, Salonga R, Tan D, et al. Evaluation of the response of Filipino COPD patients to Tiotropium (EVEREST STUDY) [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C47 Poster F78.
Diba 2009 {published data only}
-
- Diba C, Salome CM, Berend N, Harris B, Bailey D, King GG. Effect of bronchodilator on ventilation heterogeneity in COPD [Abstract]. Respirology 2009;14(Suppl 1):A19.
Diba 2011 {published data only}
-
- Diba C, King GG, Berend N, Salome CM. Improved respiratory system conductance following bronchodilator predicts reduced exertional dyspnoea. Respiratory Medicine 2011; Vol. 105, issue 9:1345‐51. - PubMed
Friedman 1998 {published data only}
-
- Friedman M, Auerbach D, Campbell S, Dunn L, Illowite J, Littner M, et al. Tiotropium in patients with COPD. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A804.
Fuhr 2010 {published data only}
-
- Fuhr R, Magnussen H, Ribera A, Kirsten AM, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide 400 µg compared with placebo and tiotropium 18 µg qd in moderate to severe COPD patients [Abstract]. Chest 2010;138(4):465A. - PubMed
-
- Fuhr R, Magnussen H, Ribera Llovera A, Kirsten A, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P1236].
Gelb 2011 {published data only}
-
- Gelb AF, Fraser C, Zamel N. Lack of protective effect of tiotropium vs induced dynamic hyperinflation in moderate COPD. Respiratory Medicine 2011;105:755‐60. - PubMed
Gurzhiy 2007 {published data only}
-
- Gurzhiy O, Pertseva T, Lykholat O. Evaluation of mucociliary clearance 's (MCC) condition in patients with COPD influence of tiotropium bromide (TB) on MCC [Abstract]. European Respiratory Journal 2007;30(Suppl 51):73s [P582].
Halpin 2006 {published data only}
-
- Halpin DMG, Menjoge S, Viel K. Tiotropium reduces the risk of COPD exacerbation irrespective of its characterization by the treatment intervention [Abstract]. European Respiratory Journal 2006;28(Suppl 50):764s [4397].
Hasani 2001 {published data only}
-
- Hasani A, Agnew J, Dilworth J, Begent L, Mier A, Sarno M, et al. Effect of tiotropium on distribution pattern of aerosol particles deposited in the lung [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A277.
-
- Hasani A, Agnew JE, Dilworth JP, Creer D, Mier A, Sarno M, et al. Effect of tiotropium on lung ventilatory distribution in COPD patients. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A282.
Hasani 2001b {published data only}
-
- Hasani A, Toms N, Creer DD, Agnew JE, Dilworth JP, Sarno M, et al. Effect of inhaled tiotropium on tracheobronchial clearance in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):245s.
Hirata 2003 {published data only}
-
- Hirata K, Nishimura M, Ichinose M, Nishimura K, Nagai A, Yoshida M, et al. Tiotropium once daily improves health status in Japanese patients with COPD [Abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:A035 Poster D79.
-
- Ichinose M, Nishimura M, Hirata K, Nagai A, Yoshida M, Fukuchi Y. Tiotropium once daily improves spirometry over 24 hours in Japanese patient with COPD [Abstract]. American Thoracic Society Meeting; 2003 May 16‐21; Seattle. 2003:A035 Poster D80.
Kerstjens 2004 {published data only}
-
- Kerstjens HAM, Bantje TA, Luursema PB, Damste HEJS, Jong JW, Lee A, et al. Tiotropium maintainence therapy and the additive effects of a single dose of ipratropium bromide or fenoterol in patient with COPD [Abstract]. American Thoracic Society 100th International Conference 2004 May 21‐26 Orlando. 2004:C22 Poster 514.
Langley 2002 {published data only}
-
- Langley S, Towse L, Kesten S, Claverley PM. Heart rate and rhythm analysis from holter monitoring in COPD patients receiving tiotropium. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A592.
McNicholas 2001 {published data only}
-
- McNicholas WT, Calverley PMA, Edwards C, Lee A. Effects of anticholinergic therapy (tiotropium) on REM‐related desaturation (SaO2) and sleep quality in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A280.
Meshcheriakova 2007 {published data only}
-
- Meshcheriakova N, Belevskiy A, Cherniak A. The role of tiotropium bromide in physical training for patients with COPD [Abstract]. European Respiratory Journal 2007;30(Suppl 51):516s [E3091].
O'Donnell 2002 {published data only}
-
- Magnussen H, O'Donnell DE, Casaburi R, Keston S, Gerken F, Flüge T. Spiriva® (tiotropium) reduces lung hyper inflation in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227.
-
- O'Donnell D, He Z, Lam M, Webb K, Flüge T, Hamilton A. Reproducibility of measurements of inspiratory capacity dyspnea intensity and exercise endurance in multicentre trials in COPD [Abstract]. European Respiratory Journal 2004;24(Suppl 48):323s.
-
- O'Donnell DE, Magnussen H, Aguilaniu B, Gerken F, Hamilton A, Flüge T. Spiriva (tiotropium) improves exercise tolerance in COPD [Abstract]. First National COPD Conference; 2003 November 14‐15; Arlington, Virginia. 2003:Abstract no: 1192.
-
- O'Donnell DE, Magnussen H, Aguilaniu B, Gerken F, Hamilton A, Flüge T. Spiriva® (Tiotropium) improves exercise tolerance in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227.
-
- O'Donnell DE, Magnussen H, Aguilaniu B, Make B, Flüge T, Hamilton A. Spiriva® (Tiotropium) reduces exertional dyspnea in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A265.
O'Donnell 2004a {published data only}
-
- O'Donnell D, Maltais F, Sciurba F, Kesten S, Hamilton A. SPIRIVA® (Tiotropium) improves symptom‐limited exercise tolerance in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:D23 Poster 207.
-
- O'Donnell D, Marciniuk D, Hernandez P, Richter K, Kesten S, Hamilton A. Spiriva® (Tiotropium) reduces lung hyperinflation at rest and during exercise in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26 2004; Orlando. 2004:D23 Poster 210.
-
- O'Donnell DE, Johnson B, Richter K, Kesten S, Hamilton A. Inspiratory capacity (IC) and dyspnea during recovery from symptom‐limited exercise in COPD patients treated with tiotropium [Abstract]. European Respiratory Journal 2004;24(Suppl 48):214s.
O'Donnell 2005 {published data only}
-
- O'Donnell D, Hamilton A, Kesten S. Influence of the exercise‐limiting symptom on the relationship between lung hyperinflation and endurance time in COPD patients treated with tiotropium [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego, California. 2005:[B23] [Poster: 502].
O'Donnell 2005a {published data only}
-
- O'Donnell D, Webb KA. The effect of tiotropium on ventilatory mechanics during exercise in COPD [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego, California. 2005:[B65] [Poster: A51].
Olson 2009 {published data only}
-
- Olson T, Baldi J, Afessa B, Scanlon P, Hulsebus M, Miller A, et al. Cardiac consequences of obstruction during exercise: benefits of bronchodilation [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A4571 [Poster #J84].
Reisner 2011 {published data only}
-
- Orevillo C, St. Rose E, Strom S, Fischer T, Golden M, Thomas M, et al. Glycopyrrolate MDI demonstrates comparable efficacy and safety to tiotropium DPI in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. European Respiratory Society Annual Congress;2011 September 24‐28; Amsterdam, The Netherlands 2011;38(55):724s [P3975].
-
- Reisner C, Fogarty C, Spangenthal S, Dunn L, Kerwin EM, Quinn D, et al. Novel combination of glycopyrrolate and formoterol MDI (GFF‐MDI) provides superior bronchodilation compared to its components administered alone, tiotropium DPI, and formoterol DPI in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A6435.
-
- Reisner C, St. Rose E, Strom S, Fischer T, Golden M, Thomas M, et al. Fixed combination of glycopyrrolate and formoterol MDI (GFF‐MDI) demonstrates superior inspiratory capacity (IC) compared to tiotropium DPI (Tio) following 7 days dosing, in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. European Respiratory Society Annual Congress; 2011 September 24‐28; Amsterdam, The Netherlands 2011;38(55):150s [P879].
Rossi 2008 {published data only}
-
- Rossi S, Gladly C, Baril J, Perrault H, Bourbeau J. COPD patients who respond to tiotropium with dyspnea relief: A proof of efficacy? [Abstract]. American Thoracic Society International Conference 2008 May 16‐21; Toronto. 2008:A648[#F15].
Schilling 2000 {published data only}
-
- Schilling JC, Witek TJ, Feifel U, Souhrada JF, Disse B. Safety and tolerability of supra‐therapeutic doses of inhaled tiotropium (TIO). European Respiratory Journal 2000;16(Suppl 31):361s.
Schurmann 2004 {published data only}
-
- Schurmann W, Schmidtmann S, Moroni P, Massey D, Qidan M. Patients prefer Respimat® Soft Mist TM inhaler to HFA‐MDI [Abstract]. Primary Care Respiratory Journal 2004;13(2):118 ABS044.
Sposato 2005 {published data only}
-
- Sposato B, Salvatore M, Giovanna MM, Alberto R, Elena C, Aldo G, et al. Formoterol or tiotropium bromide do not influence nocturnal hypoxemia in stable COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1959.
ten Hacken 2007 {published data only}
-
- Hacken NH, Haart H, Grevink R, Thompson S, Roemer W, Postma DS. Bronchodilation improves endurance but not muscular efficiency in COPD [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, California, USA. 2007:Poster #M72.
van Noord 2006 {published data only}
-
- Noord JA, Cornelissen G, Aumann JL, Platz J, Mueller A, Fogarty C. Efficacy in COPD patients of tiotropium administered via the Respimat soft mist inhaler (SMI) compared to handihaler (HH) [Abstract]. European Respiratory Journal 2006;28(Suppl 50):431s [P2516]. - PubMed
Vincken 2001 {published data only}
-
- Vincken WG, Vermiere P, Menjoge SS, Keston S, Cornelissen PJG. Maintenance of bronchodilation following tiotropium in patients with mild, moderate and severe COPD in one year clinical trials. European Respiratory Journal 2001;18(Supp 33):331s.
References to studies awaiting assessment
Gu 2007 {published data only}
-
- Gu W, Sun LH, Tan Y. Effects of tiotropium on the exercise endurance of patients with COPD. Journal Southeast University (Med. Sci. Edi.) 2007;26:332–5.
Min 2006 {published data only}
-
- Min R, Huang M, Yin KS, Fu WZ, Yu WZ. Clinical trial on tiotropium for COPD. Jiangsu Medical Journal 2006;32:1078–9.
NCT00528996 {published data only}
-
- Boehringer Ingelheim. Tiotropium (SPIRIVA®) RESPIMAT®: Evaluation of fatal events – February 2010. www.trials.boehringer‐ingelheim.com (accessed 12 July 2010). [CTG: NCT00528996; trial number: 1205.14]
Xia 2007 {published data only}
-
- Xia QP, Chen JR, Tao YJ. The curative effect of tiotropium bromide in treating stable COPD. Journal of Clinical Pulmonary Medicine 2007;12:1327–8.
Yin 2010 {published data only}
-
- Yin KS, Zhang DP, Shi Y, Sun LH, Min R, Xiao YL, et al. A randomized, double‐blind, placebo‐control study of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2010;33(7):519‐23. - PubMed
Additional references
Barr 2005
Beeh 2010
-
- Beeh KM, Beier J. The short, the long, and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐59. - PubMed
Boehringer Ingelheim 2010
-
- Boehringer Ingelheim. Tiotropium (SPIRIVA®) RESPIMAT®: Evaluation of fatal events. www.trials.boehringer‐ingelheim.com/res/trial/data/pdf/Pooled_Analysis_U10‐3255‐01.pdf 2010.
Boehringer Ingleheim Global trials database
-
- Global Clinical Trials Website of Boehringer Ingelheim . http://trials.boehringer‐ingelheim.com/.
Cates 2011
-
- Cates CJ. Safety of tiotropium. BMJ 2011;342:d2970. - PubMed
Cazzola 2008
-
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008; Vol. 31, issue 2:416‐69. - PubMed
Celli 2010
-
- Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest 2010; Vol. 137, issue 1:20‐30. - PubMed
Decramer 2011
-
- Decramer M, Molenberghs G, Liu D, Celli B, Kesten S, Lystig T, et al. Premature discontinuation during the UPLIFT study. Respiratory Medicine 2011;105:1523‐30. - PubMed
Effing 2007
Egger 1997
Fukuchi 2011
-
- Fukuchi Y, Fernandez L, Kuo HP, Mahayiddin A, Celli B, Decramer M, et al. Efficacy of tiotropium in COPD patients from Asia: A subgroup analysis from the UPLIFT trial. Respirology 2011;16:825‐35. - PubMed
GOLD 2010
-
- Global Initiative for Chronic Obstructive Lung Disease. www.goldcopd.com (accessed 14th December 2009).
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hutchinson 2010
-
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medicine Journal 2010;40(5):364‐71. - PubMed
Kaplan 2010
Kesten 2007
-
- Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE. Premature discontinuation of patients: a potential bias in COPD clinical trials. European Respiratory Journal 2007;30(5):898‐906. - PubMed
Kesten 2009
Lacasse 2006
Mauskopf 2010
-
- Mauskopf JA, Baker CL, Monz BU, Juniper MD. Cost effectiveness of tiotropium for chronic obstructive pulmonary disease: a systematic review of the evidence. Journal of Medical Economics 2010;13(3):403‐17. - PubMed
Neyt 2010
NICE 2011
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, costing report, implementing NICE guidance. www.guidance.nice.org.uk/CG101/CostingReport/pdf/English.
Oxman 1992
-
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. - PubMed
Proskocil 2005
-
- Proskocil BJ, Fryer AD. Beta2‐agonist and anticholinergic drugs in the treatment of lung disease. Proceedings of the American Thoracic Society 2005;2(4):305‐10; discussion 311‐2. - PubMed
Ram 2011
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rice 2008
-
- Rice KL, Leimer I, Kesten S, Niewoehner DE. Responses to tiotropium in African‐American and Caucasian patients with chronic obstructive pulmonary disease. Translational Research 2008;152(2):88‐94. - PubMed
SGRQ‐C manual 2008
-
- Jones P. St George's Respiratory Questionnaire for COPD Patients (SGRQ‐C). www.healthstatus.sgul.ac.uk/SGRQ_download/SGRQ‐C%20Manual%202008.pdf 2008.
Singh 2009
-
- Singh S. Review: inhaled anticholinergics increase risk of major cardiovascular events in COPD. Evidence Based Medicine 2009;14(2):42‐3. - PubMed
Singh 2011
Van der Meer 2001
WHO
-
- World Health Organization. Chronic respiratory diseases. www.who.int.
Wise 2013
-
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium respimat inhaler and the risk of death in COPD. New England Jounral of Medicine 2013;369(16):1491‐501. - PubMed
Yohannes 2011
-
- Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta‐analysis of clinically relevant outcomes. Respiratory Care 2011;56(4):477‐87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

