Ipilimumab for advanced melanoma: experience from the Spanish Expanded Access Program
- PMID: 25046550
- PMCID: PMC4457498
- DOI: 10.1097/CMR.0000000000000108
Ipilimumab for advanced melanoma: experience from the Spanish Expanded Access Program
Abstract
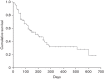
Ipilimumab, a fully human, recombinant, monoclonal antibody to cytotoxic T-lymphocyte antigen 4 improves overall survival (OS) in previously treated and untreated metastatic melanoma. This retrospective analysis reports data gathered by a questionnaire on the demographics, outcomes, and toxicity of ipilimumab administered through an Expanded Access Program (EAP). Ipilimumab 3 mg/kg was administered intravenously every 3 weeks for four cycles to adults with metastatic melanoma. Efficacy outcomes included complete response, partial response (PR), progressive disease, stabilized disease, and OS. EAP data were collected from EAP physicians. A subgroup analysis examined efficacy in elderly patients (≥70 years) and factors predictive of survival were identified. Of 355 requests for ipilimumab, resulting in 288 treatments, completed questionnaires were received for 153 ipilimumab recipients (median age 58 years, 57.2% men). Efficacy was evaluated in 144 patients: complete response in 1.3%, PR in 9.6%, PR with previous progression 8.4%, stabilized disease in 14.5%, and progressive disease in 66.2%. The median OS was 6.5 months (199 days); 1-year survival was 32.9%. Predictive survival factors included lymphocytes over 1000/ml (P=0.0008) and lactate dehydrogenase more than 1.5×upper limit of normal (P=0.003). Cutaneous, hepatic, and gastrointestinal toxicities were mild. In 30 patients aged more than 70 years, ipilimumab efficacy and tolerability was similar to that of the overall population. In the clinical practice setting, ipilimumab is effective and well tolerated in patients with advanced melanoma, including elderly patients, when administered at the recommended dosage. Ipilimumab improves treatment options for patients who, until recently, have had little hope of an improved prognosis.
Figures

References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Melanoma: Version 2 2013Washington:National Comprehensive Cancer Network; Available at: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. [Accessed 28 May 2013].
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2 Cancer incidence and mortality worldwide: IARC Cancer Base No. 10. 2010. Available at: http://globocan.iarc.fr [Accessed 28 May 2013].
-
- Gasent Blesa JM, Grande Pulido E, Alberola Candel V, Provencio Pulla M. Melanoma: from darkness to promise. Am J Clin Oncol 2011; 34:179–187. - PubMed
-
- American Cancer Society. Melanoma skin cancer 2013New York:American Cancer Society; Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003120-pdf.pdf. [Accessed 28 May 2013].
-
- Dummer R, Hauschild A, Guggenheim M, Jost L, Pentheroudakis G. ESMO Guidelines Working Group. Melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21Suppl 5v194–v197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

