Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis
- PMID: 25047515
- PMCID: PMC4165028
- DOI: 10.1124/jpet.114.216044
Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis
Abstract
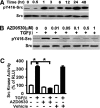
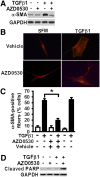
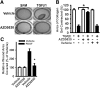
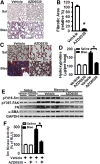
Myofibroblasts are effector cells in fibrotic disorders that synthesize and remodel the extracellular matrix (ECM). This study investigated the role of the Src kinase pathway in myofibroblast activation in vitro and fibrogenesis in vivo. The profibrotic cytokine, transforming growth factor β1 (TGF-β1), induced rapid activation of Src kinase, which led to myofibroblast differentiation of human lung fibroblasts. The Src kinase inhibitor AZD0530 (saracatinib) blocked TGF-β1-induced Src kinase activation in a dose-dependent manner. Inhibition of Src kinase significantly reduced α-smooth muscle actin (α-SMA) expression, a marker of myofibroblast differentiation, in TGF-β1-treated lung fibroblasts. In addition, the induced expression of collagen and fibronectin and three-dimensional collagen gel contraction were also significantly inhibited in AZD0530-treated fibroblasts. The therapeutic efficiency of Src kinase inhibition in vivo was tested in the bleomycin murine lung fibrosis model. Src kinase activation and collagen accumulation were significantly reduced in the lungs of AZD0530-treated mice when compared with controls. Furthermore, the total fibrotic area and expression of α-SMA and ECM proteins were significantly decreased in lungs of AZD0530-treated mice. These results indicate that Src kinase promotes myofibroblast differentiation and activation of lung fibroblasts. Additionally, these studies provide proof-of-concept for targeting the noncanonical TGF-β signaling pathway involving Src kinase as an effective therapeutic strategy for lung fibrosis.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Boggon TJ, Eck MJ. (2004) Structure and regulation of Src family kinases. Oncogene 23:7918–7927 - PubMed
-
- Cai GQ, Chou CF, Hu M, Zheng A, Reichardt LF, Guan JL, Fang H, Luckhardt TR, Zhou Y, Thannickal VJ, et al. (2012) Neuronal Wiskott-Aldrich syndrome protein (N-WASP) is critical for formation of α-smooth muscle actin filaments during myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol 303:L692–L702 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

