Natural allelic variations in glutathione peroxidase-1 affect its subcellular localization and function
- PMID: 25047527
- PMCID: PMC4167490
- DOI: 10.1158/0008-5472.CAN-14-0660
Natural allelic variations in glutathione peroxidase-1 affect its subcellular localization and function
Abstract
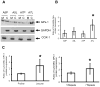
Glutathione peroxidase 1 (GPx-1) has been implicated in the etiology of several common diseases due to the association between specific allelic variations and cancer risk. The most common among these variations are the codon 198 polymorphism that results in either a leucine or proline and the number of alanine repeat codons in the coding sequence. The molecular and biologic consequences of these variations remain to be characterized. Toward achieving this goal, we have examined the cellular location of GPx-1 encoded by allelic variants by ectopically expressing these genes in MCF-7 human breast carcinoma cells that produce undetectable levels of GPx-1, thus achieving exclusive expression in the same cellular environment. A differential distribution between the cytoplasm and mitochondria was observed, with the allele expressing the leucine-198 polymorphism and 7 alanine repeats being more cytoplasmically located than the other alleles examined. To assess whether the distribution of GPx-1 between the cytoplasm and mitochondria had a biologic consequence, we engineered derivative GPx-1 proteins that were targeted to the mitochondria by the addition of a mitochondria targeting sequence and expressed these proteins in MCF-7 cells. These cells were examined for their response to oxidative stress, energy metabolism, and impact on cancer-associated signaling molecules. The results obtained indicated that both primary GPx-1 sequence and cellular location have a profound impact on cellular biology and offer feasible hypotheses about how expression of distinct GPx-1 alleles can affect cancer risk. Cancer Res; 74(18); 5118-26. ©2014 AACR.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures

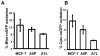




References
-
- Berry MJ, Banu L, Chen Y, Mandel SJ, Kiefer JD, Harney JW, et al. Recognition of UGA as a selenocysteine codon in Type I deiodinase requires sequences in the 3′ untranslated region. Nature. 1991;353:273–6. - PubMed
-
- Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

