Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair
- PMID: 25047629
- PMCID: PMC4140660
- DOI: 10.1016/j.biomaterials.2014.07.006
Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair
Abstract
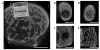
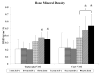
The present work investigated the use of biodegradable hydrogel composite scaffolds, based on the macromer oligo(poly(ethylene glycol) fumarate) (OPF), to deliver growth factors for the repair of osteochondral tissue in a rabbit model. In particular, bilayered OPF composites were used to mimic the structural layers of the osteochondral unit, and insulin-like growth factor-1 (IGF-1) and bone morphogenetic protein-2 (BMP-2) were loaded into gelatin microparticles and embedded within the OPF hydrogel matrix in a spatially controlled manner. Three different scaffold formulations were implanted in a medial femoral condyle osteochondral defect: 1) IGF-1 in the chondral layer, 2) BMP-2 in the subchondral layer, and 3) IGF-1 and BMP-2 in their respective separate layers. The quantity and quality of osteochondral repair was evaluated at 6 and 12 weeks with histological scoring and micro-computed tomography (micro-CT). While histological scoring results at 6 weeks showed no differences between experimental groups, micro-CT analysis revealed that the delivery of BMP-2 alone increased the number of bony trabecular islets formed, an indication of early bone formation, over that of IGF-1 delivery alone. At 12 weeks post-implantation, minimal differences were detected between the three groups for cartilage repair. However, the dual delivery of IGF-1 and BMP-2 had a higher proportion of subchondral bone repair, greater bone growth at the defect margins, and lower bone specific surface than the single delivery of IGF-1. These results suggest that the delivery of BMP-2 enhances subchondral bone formation and that, while the dual delivery of IGF-1 and BMP-2 in separate layers does not improve cartilage repair under the conditions studied, they may synergistically enhance the degree of subchondral bone formation. Overall, bilayered OPF hydrogel composites demonstrate potential as spatially-guided, multiple growth factor release vehicles for osteochondral tissue repair.
Keywords: Bone morphogenetic protein-2; Cartilage repair; Insulin-like growth factor-1; Rabbit model; Subchondral bone.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures












References
-
- Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: A crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307–14. - PubMed
-
- Danisovic L, Varga I, Polak S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell. 2012;44:69–73. - PubMed
-
- Radin EL, Ehrlich MG, Chernack R, Abernethy P, Paul IL, Rose RM. Effect of repetitive impulsive loading on knee joints of rabbits. Clin Orthop Relat Res. 1978:288–93. - PubMed
-
- Serink MT, Nachemson A, Hansson G. Effect of impact loading on rabbit knee joints. Acta Orthop Scand. 1977;48:250–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

