Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization
- PMID: 25048432
- PMCID: PMC4108220
- DOI: 10.1016/S1572-1000(05)00007-4
Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization
Abstract
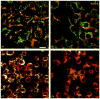
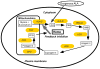
The use of non-toxic dyes or photosensitizers (PS) in combination with harmless visible light that is known as photodynamic therapy (PDT) has been known for over a hundred years, but is only now becoming widely used. Originally developed as a tumor therapy, some of its most successful applications are for non-malignant disease. In a series of three reviews we will discuss the mechanisms that operate in the field of PDT. Part one discusses the recent explosion in discovery and chemical synthesis of new PS. Some guidelines on how to choose an ideal PS for a particular application are presented. The photochemistry and photophysics of PS and the two pathways known as Type I (radicals and reactive oxygen species) and Type II (singlet oxygen) photochemical processes are discussed. To carry out PDT effectively in vivo, it is necessary to ensure sufficient light reaches all the diseased tissue. This involves understanding how light travels within various tissues and the relative effects of absorption and scattering. The fact that most of the PS are also fluorescent allows various optical imaging and monitoring strategies to be combined with PDT. The most important factor governing the outcome of PDT is how the PS interacts with cells in the target tissue or tumor, and the key aspect of this interaction is the subcellular localization of the PS. Examples of PS that localize in mitochondria, lysosomes, endoplasmic reticulum, Golgi apparatus and plasma membranes are given. Finally the use of 5-aminolevulinic acid as a natural precursor of the heme biosynthetic pathway, stimulates accumulation of the PS protoporphyrin IX is described.
Figures



References
-
- Jesionek A, von Tappenier H. Zur behandlung der hautcarcinomit mit fluorescierenden stoffen. Muench Med Wochneshr. 1903;47:2042.
-
- Hausman W. Die sensibilisierende wirkung des hematoporphyrins. Biochem Z. 1911;30:276.
-
- Figge FH, Weiland GS, Manganiello LO. Affinity of neoplastic, embryonic and traumatized tissues for porphyrins and metalloporphyrins. Proc Soc Exp Biol Med. 1948;68:640. - PubMed
-
- Lipson RL, Baldes EJ. The photodynamic properties of a particular hematoporphyin derivative. Arch Dermatol. 1960;82:508. - PubMed
-
- Dougherty TJ. Activated dyes as antitumor agents. J Natl Cancer Inst. 1974;52:1333–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

