Catalytic efficiency of designed catalytic proteins
- PMID: 25048695
- PMCID: PMC4182119
- DOI: 10.1016/j.sbi.2014.06.006
Catalytic efficiency of designed catalytic proteins
Abstract
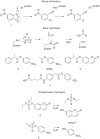
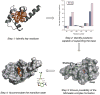
The de novo design of catalysts that mimic the affinity and specificity of natural enzymes remains one of the Holy Grails of chemistry. Despite decades of concerted effort we are still unable to design catalysts as efficient as enzymes. Here we critically evaluate approaches to (re)design of novel catalytic function in proteins using two test cases: Kemp elimination and ester hydrolysis. We show that the degree of success thus far has been modest when the rate enhancements seen for the designed proteins are compared with the rate enhancements by small molecule catalysts in solvents with properties similar to the active site. Nevertheless, there are reasons for optimism: the design methods are ever improving and the resulting catalyst can be efficiently improved using directed evolution.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
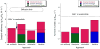
References
-
- Eliot TS. The Waste Land. New York: Horace Liveright; 1922.
-
- Kunitake T, Shinkai S. Catalysis by micelles, membranes and other aqueous aggregates as models of enzyme action. Adv Phys Org Chem. 1981;17:435–487.
-
- Breslow R, Dong SD. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem Rev. 1998;98:1997–2012. - PubMed
-
- Cram DJ. The design of molecular hosts, guest and their complexes. Angew Chem Int Ed Engl. 2003;27:1009–1020.
-
- Hilvert D. Critical analysis of antibody catalysis. Annu Rev Biochem. 2000;69:751–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

