Homer2 protein regulates plasma membrane Ca²⁺-ATPase-mediated Ca²⁺ signaling in mouse parotid gland acinar cells
- PMID: 25049230
- PMCID: PMC4155665
- DOI: 10.1074/jbc.M114.577221
Homer2 protein regulates plasma membrane Ca²⁺-ATPase-mediated Ca²⁺ signaling in mouse parotid gland acinar cells
Abstract
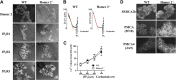
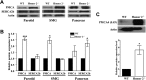
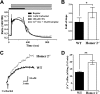
Homer proteins are scaffold molecules with a domain structure consisting of an N-terminal Ena/VASP homology 1 protein-binding domain and a C-terminal leucine zipper/coiled-coil domain. The Ena/VASP homology 1 domain recognizes proline-rich motifs and binds multiple Ca(2+)-signaling proteins, including G protein-coupled receptors, inositol 1,4,5-triphosphate receptors, ryanodine receptors, and transient receptor potential channels. However, their role in Ca(2+) signaling in nonexcitable cells is not well understood. In this study, we investigated the role of Homer2 on Ca(2+) signaling in parotid gland acinar cells using Homer2-deficient (Homer2(-/-)) mice. Homer2 is localized at the apical pole in acinar cells. Deletion of Homer2 did not affect inositol 1,4,5-triphosphate receptor localization or channel activity and did not affect the expression and activity of sarco/endoplasmic reticulum Ca(2+)-ATPase pumps. In contrast, Homer2 deletion markedly increased expression of plasma membrane Ca(2+)-ATPase (PMCA) pumps, in particular PMCA4, at the apical pole. Accordingly, Homer2 deficiency increased Ca(2+) extrusion by acinar cells. These findings were supported by co-immunoprecipitation of Homer2 and PMCA in wild-type parotid cells and transfected human embryonic kidney 293 (HEK293) cells. We identified a Homer-binding PPXXF-like motif in the N terminus of PMCA that is required for interaction with Homer2. Mutation of the PPXXF-like motif did not affect the interaction of PMCA with Homer1 but inhibited its interaction with Homer2 and increased Ca(2+) clearance by PMCA. These findings reveal an important regulation of PMCA by Homer2 that has a central role on PMCA-mediated Ca(2+) signaling in parotid acinar cells.
Keywords: Calcium ATPase; Calcium Transport; Cell Signaling; Homer Proteins; Parotid Gland; Plasma Membrane Ca2+-ATPase; Proline-rich Motif; Protein-Protein Interaction; Scaffold Protein.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 - PubMed
-
- Petersen O. H., Tepikin A. V. (2008) Polarized calcium signaling in exocrine gland cells. Annu. Rev. Physiol. 70, 273–299 - PubMed
-
- Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 - PubMed
-
- Fagni L., Chavis P., Ango F., Bockaert J. (2000) Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 23, 80–88 - PubMed
-
- Szumlinski K. K., Kalivas P. W., Worley P. F. (2006) Homer proteins: implications for neuropsychiatric disorders. Curr. Opin. Neurobiol. 16, 251–257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

