Targeting potassium channels in cancer
- PMID: 25049269
- PMCID: PMC4107787
- DOI: 10.1083/jcb.201404136
Targeting potassium channels in cancer
Abstract
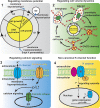
Potassium channels are pore-forming transmembrane proteins that regulate a multitude of biological processes by controlling potassium flow across cell membranes. Aberrant potassium channel functions contribute to diseases such as epilepsy, cardiac arrhythmia, and neuromuscular symptoms collectively known as channelopathies. Increasing evidence suggests that cancer constitutes another category of channelopathies associated with dysregulated channel expression. Indeed, potassium channel-modulating agents have demonstrated antitumor efficacy. Potassium channels regulate cancer cell behaviors such as proliferation and migration through both canonical ion permeation-dependent and noncanonical ion permeation-independent functions. Given their cell surface localization and well-known pharmacology, pharmacological strategies to target potassium channel could prove to be promising cancer therapeutics.
© 2014 Huang and Jan.
Figures


References
-
- Bi, D., Toyama K., Lemaître V., Takai J., Fan F., Jenkins D.P., Wulff H., Gutterman D.D., Park F., and Miura H.. 2013. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J. Biol. Chem. 288:15843–15853 10.1074/jbc.M112.427187 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

