Endothelial cell protein C receptor: a multiliganded and multifunctional receptor
- PMID: 25049281
- PMCID: PMC4155268
- DOI: 10.1182/blood-2014-05-578328
Endothelial cell protein C receptor: a multiliganded and multifunctional receptor
Abstract
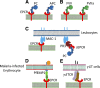
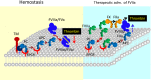
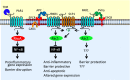
Endothelial cell protein C receptor (EPCR) was first identified and isolated as a cellular receptor for protein C on endothelial cells. EPCR plays a crucial role in the protein C anticoagulant pathway by promoting protein C activation. In the last decade, EPCR has received wide attention after it was discovered to play a key role in mediating activated protein C (APC)-induced cytoprotective effects, including antiapoptotic, anti-inflammatory, and barrier stabilization. APC elicits cytoprotective signaling through activation of protease activated receptor-1 (PAR1). Understanding how EPCR-APC induces cytoprotective effects through activation of PAR1, whose activation by thrombin is known to induce a proinflammatory response, has become a major research focus in the field. Recent studies also discovered additional ligands for EPCR, which include factor VIIa, Plasmodium falciparum erythrocyte membrane protein, and a specific variant of the T-cell receptor. These observations open unsuspected new roles for EPCR in hemostasis, malaria pathogenesis, innate immunity, and cancer. Future research on these new discoveries will undoubtedly expand our understanding of the role of EPCR in normal physiology and disease, as well as provide novel insights into mechanisms for EPCR multifunctionality. Comprehensive understanding of EPCR may lead to development of novel therapeutic modalities in treating hemophilia, inflammation, cerebral malaria, and cancer.
© 2014 by The American Society of Hematology.
Figures




References
-
- Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269(42):26486–26491. - PubMed
-
- Oganesyan V, Oganesyan N, Terzyan S, et al. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem. 2002;277(28):24851–24854. - PubMed
-
- López-Sagaseta J, Puy C, Tamayo I, et al. sPLA2-V inhibits EPCR anticoagulant and antiapoptotic properties by accommodating lysophosphatidylcholine or PAF in the hydrophobic groove. Blood. 2012;119(12):2914–2921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

