Blockade of oncogenic IκB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors
- PMID: 25049379
- PMCID: PMC4128109
- DOI: 10.1073/pnas.1411701111
Blockade of oncogenic IκB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors
Abstract
In the activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL), NF-κB activity is essential for viability of the malignant cells and is sustained by constitutive activity of IκB kinase (IKK) in the cytoplasm. Here, we report an unexpected role for the bromodomain and extraterminal domain (BET) proteins BRD2 and BRD4 in maintaining oncogenic IKK activity in ABC DLBCL. IKK activity was reduced by small molecules targeting BET proteins as well as by genetic knockdown of BRD2 and BRD4 expression, thereby inhibiting downstream NF-κB-driven transcriptional programs and killing ABC DLBCL cells. Using a high-throughput platform to screen for drug-drug synergy, we observed that the BET inhibitor JQ1 combined favorably with multiple drugs targeting B-cell receptor signaling, one pathway that activates IKK in ABC DLBCL. The BTK kinase inhibitor ibrutinib, which is in clinical development for the treatment of ABC DLBCL, synergized strongly with BET inhibitors in killing ABC DLBCL cells in vitro and in a xenograft mouse model. These findings provide a mechanistic basis for the clinical development of BET protein inhibitors in ABC DLBCL, particularly in combination with other modulators of oncogenic IKK signaling.
Keywords: cancer therapy; drug synergism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
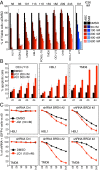
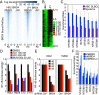

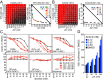
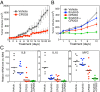
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- SRA/SRP043524
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

