Prolactin prevents hepatocellular carcinoma by restricting innate immune activation of c-Myc in mice
- PMID: 25049387
- PMCID: PMC4128121
- DOI: 10.1073/pnas.1404267111
Prolactin prevents hepatocellular carcinoma by restricting innate immune activation of c-Myc in mice
Abstract
Women are more resistant to hepatocellular carcinoma (HCC) than men despite equal exposure to major risk factors, such as hepatitis B or C virus infection. Female resistance is hormone-dependent, as evidenced by the sharp increase in HCC incidence in postmenopausal women who do not take hormone replacement therapy. In rodent models sex-dimorphic HCC phenotypes are pituitary-dependent, suggesting that sex hormones act via the gonadal-hypophyseal axis. We found that the estrogen-responsive pituitary hormone prolactin (PRL), signaling through hepatocyte-predominant short-form prolactin receptors (PRLR-S), constrained TNF receptor-associated factor (TRAF)-dependent innate immune responses invoked by IL-1β, TNF-α, and LPS/Toll-like receptor 4 (TLR4), but not TRIF-dependent poly(I:C)/TLR3. PRL ubiquitinated and accelerated poststimulatory decay of a "trafasome" comprised of IRAK1, TRAF6, and MAP3K proteins, abrogating downstream activation of c-Myc-interacting pathways, including PI3K/AKT, mTORC1, p38 MAPK, and NF-κB. Consistent with this finding, we documented exaggerated male liver responses to immune stimuli in mice and humans. Tumor promotion through, but regulation above, the level of c-Myc was demonstrated by sex-independent HCC eruption in Alb-Myc transgenic mice. PRL deficiency accelerated liver carcinogenesis in Prl(-/-) mice of both sexes. Conversely, pharmacologic PRL mobilization using the dopamine D2 receptor antagonist domperidone prevented HCC in tumor-prone C3H/HeN males. Viewed together, our results demonstrate that PRL constrains tumor-promoting liver inflammation by inhibiting MAP3K-dependent activation of c-Myc at the level of the trafasome. PRL-targeted therapy may hold promise for reducing the burden of liver cancer in high-risk men and women.
Keywords: innate immunity; lactotrophs; liver neoplasms; sex dimorphism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
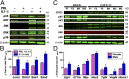
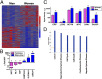

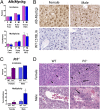
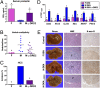

Comment in
-
Hepatocellular carcinoma: gender differences.Nat Rev Cancer. 2014 Sep;14(9):578. doi: 10.1038/nrc3808. Epub 2014 Aug 14. Nat Rev Cancer. 2014. PMID: 25118603 No abstract available.
References
-
- Mucci LA, et al. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. BJOG. 2001;108(3):291–294. - PubMed
-
- Yu MW, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38(6):1393–1400. - PubMed
-
- Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

