Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes
- PMID: 25049397
- PMCID: PMC4128148
- DOI: 10.1073/pnas.1317022111
Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes
Abstract
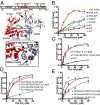
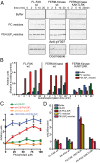
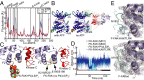
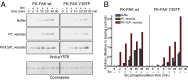
Focal adhesion kinase (FAK) is a nonreceptor tyrosine kinase (NRTK) with key roles in integrating growth and cell matrix adhesion signals, and FAK is a major driver of invasion and metastasis in cancer. Cell adhesion via integrin receptors is well known to trigger FAK signaling, and many of the players involved are known; however, mechanistically, FAK activation is not understood. Here, using a multidisciplinary approach, including biochemical, biophysical, structural, computational, and cell biology approaches, we provide a detailed view of a multistep activation mechanism of FAK initiated by phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2]. Interestingly, the mechanism differs from canonical NRTK activation and is tailored to the dual catalytic and scaffolding function of FAK. We find PI(4,5)P2 induces clustering of FAK on the lipid bilayer by binding a basic region in the regulatory 4.1, ezrin, radixin, moesin homology (FERM) domain. In these clusters, PI(4,5)P2 induces a partially open FAK conformation where the autophosphorylation site is exposed, facilitating efficient autophosphorylation and subsequent Src recruitment. However, PI(4,5)P2 does not release autoinhibitory interactions; rather, Src phosphorylation of the activation loop in FAK results in release of the FERM/kinase tether and full catalytic activation. We propose that PI(4,5)P2 and its generation in focal adhesions by the enzyme phosphatidylinositol 4-phosphate 5-kinase type Iγ are important in linking integrin signaling to FAK activation.
Keywords: cell signaling; phosphoinositides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

