Immobilization of the Antarctic Bacillus sp. LX-1 α-Galactosidase on Eudragit L-100 for the Production of a Functional Feed Additive
- PMID: 25049822
- PMCID: PMC4093379
- DOI: 10.5713/ajas.2012.12557
Immobilization of the Antarctic Bacillus sp. LX-1 α-Galactosidase on Eudragit L-100 for the Production of a Functional Feed Additive
Abstract
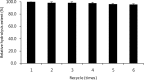
Partially purified α-galactosidase from Bacillus sp. LX-1 was non-covalently immobilized on a reversibly soluble-insoluble polymer, Eudragit L-100, and an immobilization efficiency of 0.93 was obtained. The optimum pH of the free and immobilized enzyme was 6.5 to 7.0 and 7.0, respectively, while there was no change in optimum temperature between the free and immobilized α-galactosidase. The immobilized α-galactosidase was reutilized six times without significant loss in activity. The immobilized enzyme showed good storage stability at 37°C, retaining about 50% of its initial activity even after 18 d at this temperature, while the free enzyme was completely inactivated. The immobilization of α-galactosidase from Bacillus sp. LX-1 on Eudragit L-100 may be a promising strategy for removal of α-galacto-oligosaccharides such as raffinose and stachyose from soybean meal and other legume in feed industry.
Keywords: Bacillus; Eudragit L-100; Feed Industry; Immobilization; α-Galacto-oligosaccharides; α-Galactosidase.
Figures





References
-
- Ai Z, Jiang Z, Li L, Deng W, Kusakabe I, Li H. Immobilization of Streptomyces olivaceoviridis E-86 xylanase on Eudragit S-100 for xylo-oligosaccharide production. Process Biochem. 2005;40:2707–2714.
-
- Anderson RL, Wolf WJ. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J Nutr. 1995;125:581S–588S. - PubMed
-
- Bayraktar H, Serilmez M, Karkas T, Celem EB, Onal S. Immobilization and stabilization of α-galactosidase on Sepabeads EC-EA and EC-HA. Int J Biol Macromol. 2011;49:855–860. - PubMed
-
- Bora U, Kannan K, Nahar P. A simple method for functionalization of cellulose membrane for covalent immobilization of biomolecules. J Membr Sci. 2005;250:215–222.
-
- Celem EB, Onal S. Immobilization of phytase on epoxy-activated Sepabead EC-EP for the hydrolysis of soymilk phytate. J Mol Cat B: Enzym. 2009;61:150–156. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

