Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer's disease
- PMID: 25051175
- PMCID: PMC4106886
- DOI: 10.1371/journal.pone.0103067
Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer's disease
Abstract
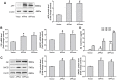
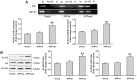
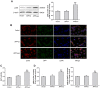
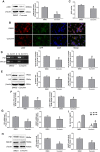
Epigenetic modifications, particularly histone acetylation, have been implicated in Alzheimer's disease (AD). While previous studies have suggested that histone hypoacetylation may regulate the expression of genes associated with memory and learning in AD, little is known about histone regulation of AD-related genes such as Presenilin 1(PS1) and beta-site amyloid precursor protein cleaving enzyme 1(BACE1). By utilizing neuroblastoma N2a cells transfected with Swedish mutated human amyloid precursor protein (APP) (N2a/APPswe) and wild-type APP (N2a/APPwt) as cellular models of AD, we examined the alterations of histone acetylation at the promoter regions of PS1 and BACE1 in these cells. Our results revealed that histone H3 acetylation in PS1 and BACE1 promoters is markedly increased in N2a/APPswe cells when compared to N2a/APPwt cells and control cells (vector-transfected), respectively, causing the elevated expression of PS1 and BACE1. In addition, expression of histone acetyltransferase (HAT) adenoviral E1A-associated 300-kDa protein (p300) is dramatically enhanced in N2a/APPswe cells compared to N2a/APPwt and control cells. We have further demonstrated the direct binding of p300 protein to the PS1 and BACE1 promoters in N2a/APPswe cells. The expression levels of H3 acetylation of the PS1 and BACE1 promoters and p300 protein, however, were found to be not significantly different in N2a/APPwt cells when compared to controls in our studies. Furthermore, curcumin, a natural selective inhibitor of p300 in HATs, significantly suppressed the expression of PS1 and BACE1 through inhibition of H3 acetylation in their promoter regions in N2a/APPswe cells. These findings indicated that histone acetyltransferase p300 plays a critical role in controlling the expression of AD-related genes through regulating the acetylation of their promoter regions, suggesting that p300 may represent a novel potential therapeutic target for AD.
Conflict of interest statement
Figures
Similar articles
-
Histone deacetylase inhibitors VPA and WT161 ameliorate the pathological features and cognitive impairments of the APP/PS1 Alzheimer's disease mouse model by regulating the expression of APP secretases.Alzheimers Res Ther. 2024 Jan 20;16(1):15. doi: 10.1186/s13195-024-01384-0. Alzheimers Res Ther. 2024. PMID: 38245771 Free PMC article.
-
Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease.J Biol Chem. 2010 Sep 3;285(36):27737-44. doi: 10.1074/jbc.M110.117960. Epub 2010 Jul 1. J Biol Chem. 2010. PMID: 20595388 Free PMC article.
-
Swedish mutant APP-based BACE1 binding site peptide reduces APP β-cleavage and cerebral Aβ levels in Alzheimer's mice.Sci Rep. 2015 Jun 19;5:11322. doi: 10.1038/srep11322. Sci Rep. 2015. PMID: 26091071 Free PMC article.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Brain insulin resistance linked Alzheimer's and Parkinson's disease pathology: An undying implication of epigenetic and autophagy modulation.Inflammopharmacology. 2023 Apr;31(2):699-716. doi: 10.1007/s10787-023-01187-z. Epub 2023 Mar 23. Inflammopharmacology. 2023. PMID: 36952096 Review.
-
Alzheimer's Disease-Related Epigenetic Changes: Novel Therapeutic Targets.Mol Neurobiol. 2024 Mar;61(3):1282-1317. doi: 10.1007/s12035-023-03626-y. Epub 2023 Sep 12. Mol Neurobiol. 2024. PMID: 37700216 Review.
-
The Essential Medicinal Chemistry of Curcumin.J Med Chem. 2017 Mar 9;60(5):1620-1637. doi: 10.1021/acs.jmedchem.6b00975. Epub 2017 Jan 11. J Med Chem. 2017. PMID: 28074653 Free PMC article. Review.
-
Protective effect of Gastrodia elata Blume in a Caenorhabditis elegans model of Alzheimer's disease based on network pharmacology.Biomed Rep. 2023 Apr 12;18(5):37. doi: 10.3892/br.2023.1620. eCollection 2023 May. Biomed Rep. 2023. PMID: 37113386 Free PMC article.
-
"Curcumin, the King of Spices": Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases.Curr Pharmacol Rep. 2015 Apr;1(2):129-139. doi: 10.1007/s40495-015-0018-x. Epub 2015 Jan 30. Curr Pharmacol Rep. 2015. PMID: 26457241 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

